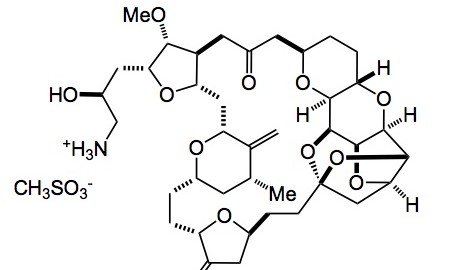
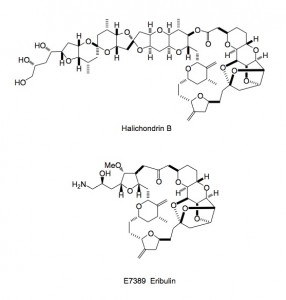
Not too much going on this week in the ASAP world, with the holiday season upon us; so although I only recently started an ASAP of the week I have a personal choice for a publication of the year. It is the description of the optimisation and scale-up of Halaven or E7389 eribulin mesylate a compound derived from halichondrin B.
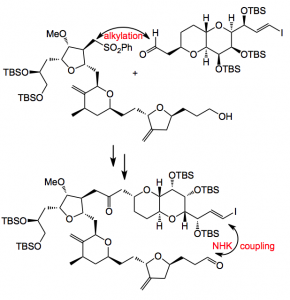
 Firstly a review written by Melvin J. Yu, Wanjun Zheng and Boris M. Seletsky of Eisai USA covering the work between 1993-2002 and secondly a report on the scale-up of the final steps. The synthesis involves several Nozaki-Hiyama-Kishi (NHK) coupling reactions. This involves the use of chromium (II) chloride in the presence of nickel (II) chloride and adds a vinyl iodide efficiently to an aldehyde. The review article nicely describes the progression from micrograms to grams of E7389 starting from the simple starting materials.
Firstly a review written by Melvin J. Yu, Wanjun Zheng and Boris M. Seletsky of Eisai USA covering the work between 1993-2002 and secondly a report on the scale-up of the final steps. The synthesis involves several Nozaki-Hiyama-Kishi (NHK) coupling reactions. This involves the use of chromium (II) chloride in the presence of nickel (II) chloride and adds a vinyl iodide efficiently to an aldehyde. The review article nicely describes the progression from micrograms to grams of E7389 starting from the simple starting materials.
The report deals with the end game, the coupling of two advanced intermediates, followed by the progression through to the final product. The assembly of these two fragments sets the stage for the completion of the macrocyclic framework via a NHK reaction.
Around 1 Kg of each fragment was used in the alkylation step, this led to 75-95% yield of the mixture of diastereoisomers. Further manipulation delivered the vinyl iodide/aldehyde for the NHK cyclisation. This was carried out on a 1Kg scale and produced the cyclised compound (allylic alcohol) in about 80% yield. This reaction required 0.789Kg of CrCl2 quantities that are really quite difficult to obtain in the required quality.
The authors put it perfectly “In summary, completion of the synthesis of Halaven® reproducibly in multigram quantities was enabled by: (i) effective supply chain management providing high quality and sufficient quantities of the (C14–C35) fragment and the (C1– C13) fragment, (ii) efficient sulfone–aldehyde coupling aided by controlled dianion formation and optimized nucleophilic addition over proton transfer in a nonpolar solvent, (iii) chemoselective samarium iodide mediated sulfone removal in the presence of aldehyde and vinyl iodide moieties, (iv) efficient Ni(II)/Cr(II)-mediated macrocyclization under non-high-dilution conditions facilitated by inverse addition of substrate to a soluble organometallic species using the asymmetric version of the NHK reaction, (v) controlled formation and crystallization of the polycyclic caged penultimate diol 2, and (vi) selective amino alcohol formation and generation of eribulin mesylate. The overall commercial process to Halaven® underscores the power of the Nozaki–Hiyama–Kishi reaction to realize the construction of structurally complex molecules with human health care impact. “
The amount of the free base synthesised (about 250g) can be found in the supporting material to the report as well as detailed experimental conditions. Obviously the reaction sequence has been carried out on larger scale, the exact amounts will probably not be revealed as is the norm for the industry. To my way of thinking there are too many heavy metals involved, especially in the end-game, but I’m sure that the development crew at Eisai have this under control along with their QA department. I agree completely with their statement above. It is a tremendous example of the power of organic synthesis in being able to synthesise such complex molecules on a commercial basis. I’m sure the development team had a lot of fun, filled with scary moments, however looking back I bet they enjoyed this whole project.
![]()