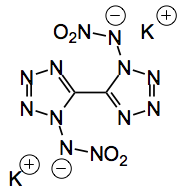
Prof. Klapötke and his group are at it again, with an even more set of impressive explosive results. This time they have synthesised potassium 1,1′-Dinitro-5,5′-bistetrazolate (I will call it K(a)boom).
It is easily synthesised from dimethyl carbonate in 7 steps in about 12% yield. The reactions themselves are scary; using hydrazine, sodium azide, HCl, and nitration with N2O5!
It is hoped that this compound will replace lead azide and the like, due to the toxicity of the latter. K(a)boom is not toxic and did not kill the luminous bacteria Vibrio fischeri and the EC50 was higher than 1.4 grams per liter. So unless it explodes the bacteria survive!
Several tests were performed on this compound and I encourage you to take a look at the free supplementary material here where Prof. Klapötke kindly provided videos of the said tests, quote “Owing to their good flowability, both compounds were simply poured into the steel block and ignited unpressed. The indentation is 1.4mm, whereas lead azide reaches only 0.2 mm, using 500 mg of each compound.” K(a)boom is thermally stable, nothing happens to it when kept under isothermal conditions at 100°C. But this is what happens when you wave a flame at it:
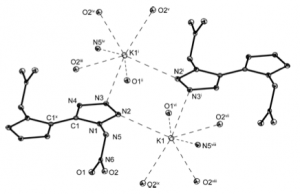
The detonation is instant. The compound was characterised by low temperature x-ray. As you see it is flat, apart from the nitro groups above and below the plane.
It is better than lead azide in all the usual measurements carried out for such compounds. It has a detonation pressure of 8330 ms, lead azide only has 5920. The gas volume released is 489 Lkg compared to 252 for lead azide.
So we have here a much greener explosive which is easy to synthesise and is non toxic, what more could you need? And just in time for the 4th July! These are one brave group of people, congratulations.
3,657 total views, no views today