A few figures relating to the synthesis of the 3d compound will help illustrate the magnitude of this synthetic effort. It began at the end of July (first reaction started in pilot plant), and finished 20 months later (compound released for human use). This is 20 months equates to about two weeks per step! The campaign was not run in an overlapping mode but rather progressed in a linear fashion. Therefore, there is a lot of scope to obtain a quicker lead time; probably one could prepare the final product in around 12 months. Around 100 persons were involved. This figure contains all laboratory and pilot plant personnel. The analytic effort is not included, but as an estimate around 10 more could be added.
The amount of product produced would equate to around 3000kg of sponge (from which it was isolated) a quantity that probably does not exist! The total number of steps is 36 with an overall yield of 0.2% (main chain). This is on the low side, but it should be remembered that the yields for each step were not been optimised. The description of the various optimisation processes thus far, in these posts, have only related to the various scale-up and reproducibility issues not to obtaining the maximum possible yield, so there is plenty of scope for increasing the yield. There were, in this “large scale synthesis”, some 18 chromatographic purifications. This has now been somewhat reduced to 15. The number of crystalline intermediates stands currently at 7.
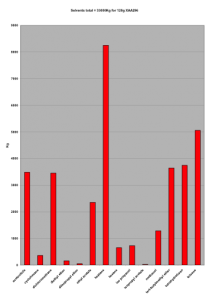
The synthesis described here will probably never be the one of choice for chemical production. Two examples suffice to illustrate this: For the preparation of 60g of drug substance an estimated 300tons of solvent per kg of final product was used, mainly heptane, toluene, acetonitrile, THF, TBME and dichloromethane, lovely green solvents.
If one assumes a yearly supply requirement for 50kg of drug substance this would involve around 50000kg of the starting intermediates to be produced! Thus supply and logistical problems are programmed in. However, there is extensive experience with this route and the problematic steps are easy to identify but perhaps less easy to optimise. Even then the amount of solvent used would have to be massively reduced.
One major problem associated with a synthesis of this length is the proper laboratory examination of the later reactions in the sequence. Initially there are no answers to these supply problems; one just has to run the small scale reaction and hope that on transfer to larger scale the reaction proceeds as expected. We certainly stumbled over this exact point during the final aldol coupling and as a result the project very nearly ended in failure, or at least the required amount of drug substance would not have been able to be delivered.
The next task is to design and scale-up a shorter more efficient alternative synthesis. Such a synthesis should eliminate all the problematic reagents and reactions along the lines described here. It should have the minimum number of chromatographic purifications and the maximum number of crystalline intermediates. High dilution reactions must be avoided in order to maximise reactor efficiency. Complex chemistry, for example aldol reactions, stereochemical introduction (alkylation, etc), should be placed at the beginning or in the middle of the synthesis, the final few steps leading to the final product need to be kept as simple as possible, for example, simple reductions or protecting group manipulations.
3,161 total views, 1 views today