Last year the Fujita group published a wonderful novel way of obtaining X-ray structures allowing structure determination on a nanogram to microgram scale. This was achieved by soaking tiny crystals of a porous complex in a solution of the target molecule. The complexes absorb the molecules of the compound whose X-ray is to be determined. This alone is a great achievement, but Prof. Fujita combined this method with HPLC thus allowing the direct structure determination of chromatographically separated compounds. Just imagine what the bothers Bragg would say to this method. Never mind them, I wish I had had this methodology it would have saved a lot of crystallising hardship. So you do your reaction HPLC it, after work-up, and get a direct structure out. I wonder if the natural product isolationists have picked up on this method? If not they should.
Prof. Buchwald has picked it up! In a recent paper he successfully applied this structural determination method to solve the structure of hypervalent iodine reagents for trifluorothiomethylation. Shen and his co-workers introduced a new reagent to electrophillically add the -SCF3 group to β-ketoesters. Shen proposed the following structure (1) for the reagent:
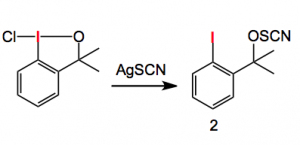
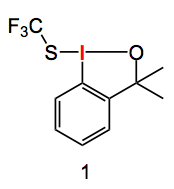 This compound belongs to a class of substances which are capable to transferring the trifluoromethyl-X- group (where X is O, S, CN, F. etc) to appropriate substrates. This is, of course, a substitution often employed in medicinal chemistry. Prof. Buchwald noticed that when the following reaction was carried out the expected product (structure type 1 above) was not obtained. The ring opened, benzyl alcohol, compound (2) was obtained:
This compound belongs to a class of substances which are capable to transferring the trifluoromethyl-X- group (where X is O, S, CN, F. etc) to appropriate substrates. This is, of course, a substitution often employed in medicinal chemistry. Prof. Buchwald noticed that when the following reaction was carried out the expected product (structure type 1 above) was not obtained. The ring opened, benzyl alcohol, compound (2) was obtained:
The structure of compound 2 was suggested by the usual analytical methods. But more interestingly the structure was confirmed using the sponge X-ray technique. So instead of crystallising you soak. The process takes about two weeks, replacing the solvent every day, much easier than crystallisation!
Both papers mentioned above deserve reading. It should also be noted , as put by Prof. Buchwald, “Importantly, whereas these results do not affect the chemical reactivity studies utilising reagent 1, as recently reported by Shen and co-workers, they do suggest that other mechanistic pathways for electrophilic trifluoromethylthiolation are possible.”
6,527 total views, 1 views today