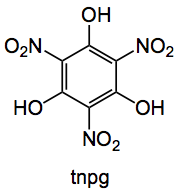
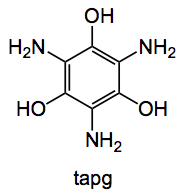
This week’s choice is the result of a collaboration between the Kappe group at the University of Graz, Austria and Bauser of Bayer’s global drug discovery in Berlin. It deals with the nitration and hydrogenation to synthesise triaminophloroglucinol (tapg):
Why is this compound of interest? Well complete hydrogenation produces a 1,3,5-triamino-1,3,5-tridoexy-cis-inositol, this compound is of importance as a ligand for dealing with metal overload, and acts as a chelating agent. For example in lead poisoning or iron overload. Such compounds are also used for the preparation of paramagnetic contrast agents. So large scale manufacture of tapg is extremely important. Now a very efficient way of making amino groups is by hydrogenation of nitro substituents over a platinum oxide catalyst, thus a viable starting material for tapg is the corresponding tri-nitro compound (tnpg) obtained by nitration of cheap phlorogluconol.:
Now you may recognise a certain similarity between tnpg and tnt! Indeed tnpg has very similar explosive tendencies to tnt. Combine this with nitration, which is a very energetic reaction and you have a slight problem which puts a dampener on any large scale batch preparation or use of tnpg.
Not to be outdone our intrepid group(s) realised that flow methods are demonstrated safe alternatives for the synthesis of nitro compounds and the efficient heat transfer shown in microfluidic apparatus can easily control the exothermic nitration reaction. A two feed system is normally used for nitrations, where the feeds are mixed in a thermostatted mircomixing unit followed by spending time in a reaction chamber. Hydrogenations are also inherently dangerous on large scale and are quite suited to flow reactors systems. So reported is a flow system in which the generated tnpg is generated and immediately hydrogenated
Various methods are used for nitration, this paper chose a mixture of a nitrate salt in sulfuric acid which are safer than the usual nitric/sulfuric mixtures and you don’t get any NOx gases formed as a nice side effect. It’s still scary as they noted for a small scale batch reaction “The reaction mixture was then stirred at room temperature. After about 5 min, precipitation of copious amounts of a yellow solid corresponding to TNPG was observed. HPLC analysis revealed that the reaction was completed within 10 min. It should be noted that, when the solutions containing the reactants were mixed without external cooling, a significant exotherm was observed, and at 3 mmol scale, the temperature of the solution increased approximately 30 °C within 5 s. Clearly, batch experiments on larger scale could lead to dangerous temperature levels with a high risk of explosion.” The flow system was established by pumping the two reactands with glass syringe pumps through a 75μL a mixing coil and into a larger residence coil immersed in a thermostated ultrasonic bath, this set-up avoids any tendency of tnpg to precipitate out. A third feed of water quenched the reaction and dissolved the solid tnpg ready for the hydrogenation step. Residence times were around 10 minutes. Using a temperature of 40°C they achieved a 98% conversion of phloroglucinol to tnpg. the other 1%s being starting materials and mono-nitrated compound.
Hydrogenation was performed using a commercial apparatus and achieved in 98% at 50°C using sulfuric acid methanol as a solvent. This produces tapg as a sulfate salt in 92% yield after crystallisation. This material is stable and can be stored in contrast to the free amine which is unstable. Further optimisation of the chemistry and the flow system allowed an overall throughput of 2 mmol/hour and produced a 82% overall yield of tapg.
Some simple chemistry but complicated process problems here which have been elegantly solved using flow techniques. This paper is a good read and contains vital information for anyone carrying out this sort of chemistry in the lab. It shows the importance of knowning exactly what goes on during a reaction. It demonstrates the proper use of analytical methods and highlights the requirement for crystallisation as a wonderful method of purification. A 3 mmol reaction scale is not beyond the academic reach and their work would only benefit from such a reaction scale much more than the μmol procedures I recently discussed here. At this scale you can at least measure the yield accurately or at least more accurately than using NMR (manipulated or not as the case may be).
5,374 total views, no views today