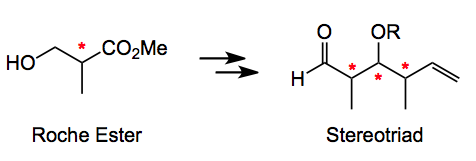
The Roche ester, in both its enantiomeric forms has, been applied as a starting point for many natural product synthesis containing stereotriads. It was developed by Roush some years ago to address the acyclic dipropionate problem.
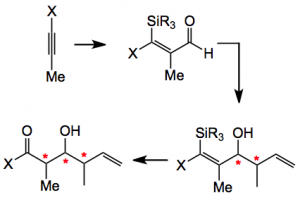
The ester and its derivatives undergo a very efficient reaction with matched chiral reagents producing products with very high diastereoselectivity. A recent paper by C. N. Foley and J. L. Leighton from Columbia University in New York discusses their approach to stereotriad synthesis not employing Roche ester thus avoiding the cost of purchasing a pure chiral compound. Their route is a three step process involving silylformylation of an acetylene followed by crotylation, and finally oxidation.
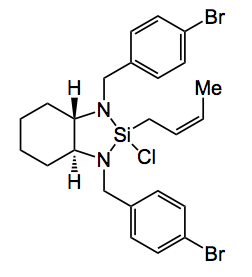
The sequence begins with the silylformylation of an acetylene. This is carried out by employing 500 psi of carbon monoxide in the presence of a rhodium catalyst at 60°C. The success of this reaction is dependant on the correct choice of silane and the silylated product must be amenable to the final oxidation step. After considerable experimentation including reaction solvent isopropoxydiphenylsilane was found to be optimal. Crotylation of the carbonylation reaction mixture with (S)(S)-cis-EZ-CrotylMix provides the cyclised product, shown below, in good yield (70% overall) and 93%ee.
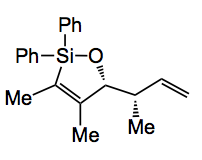 CrotylMix is a mixture of the chlorosilane, shown below, with scandium triflate in a molar ratio of 25:1.
CrotylMix is a mixture of the chlorosilane, shown below, with scandium triflate in a molar ratio of 25:1.
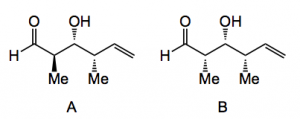
The final step is an oxidation of the cyclic silyloxy compound which goes under the name of the Tamao oxidation. This can be carried with aqueous peroxide/carbonate to give diastereoisomer A or non-aqueous (a hydroquinone) to give diastereoisomer B.
These are produced in reasonable yield and diastereoselectivity. The exact mechanism of this difference in cleavage producing the anti or syn diastereoisomer is not known with any accuracy, but it is interesting to note. The authors conclude “We have developed a new synthesis of polypropionate stereotriad building blocks that we contend represents a significant conceptual and practical advance relative to the now classical and still widely used Roche ester strategy. The synthesis proceeds in just three steps and two pots, employs exceedingly simple starting materials….. In contrast to the Roche ester approach and all other related approaches in which the α-methyl stereocenter is either purchased or synthesised by external asymmetric induction (thereby adding steps and expense), the α-methyl stereocenter is established after the crotylation event using internal diastereochemical control and is therefore obtained for “free.” “.
I would only partially agree with this statement. There is still an immense amount of work required to make this into a really viable reproducible process with the high demands on yield and diastereoselectivity. Think about scaling this up, using carbon monoxide is certainly problematic especially at those pressures. This compound has a short term exposure limit of 200ppm and a time weighted exposure of 30ppm (normal 8-hour work day and 40-hour work week). So, at least, in a “normal pilot plant” environment this may be a serious obstacle to overcome in terms of worker safety and exhaust gas limits extensive, expensive monitoring is therefore required. Also low molecular weight acetylenes can be problematic, not just because of their reactivity but their propensity to readily form explosive heavy metal salts. The CrotylMix is an interesting reagent and I am not sure about it’s bulk availability although I’m sure Aldrich would make you a few kilos or more if the price is right. As this is thrown into the formylation reaction mixture (in lieu of a work-up) there may be a possibility of forming scandium acetylide. If this exists it may or may not be explosive, I suppose one would have to make it and find out. However, it calls for glass equipment at the least to avoid any such possibility. I’m also not convinced by the cost argument. This route will be just as, if not more expensive, than the Roche ester approach.
It is the job of a process chemist to take such new methodology and get it working safely on scale, and I’m sure this would be a great challenge. I think that I’ll file this away under useful synthetic transformations and stick to Roche ester for the time being. The publication is packed full of information and is a worthy extension of this group’s previous work.
6,705 total views, 1 views today