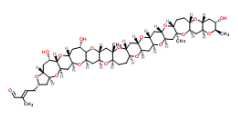
So a Friday the 13th! I hope you are not superstitious but in this week’s ASAP there are a couple of mystical things, at least I think they are. It’s from the group of Mori (looking very Regal at his desk) at Nagoya telling of their total synthesis of Gymnocin-A. Now I’m not going to draw it but here is the InChiKey: SHYKGRAJWKSPPP-BLASZRAGSA-N. If you want to see the structure it is here. Its one of these polycyclic ethers isolated from the toxic “red tides”. It has 14 contiguously fused rings and 31 stereocenters giving it at least 2,147,483,648 stereoisomers (double bond not included).
So Mori and his group synthesised this in a convergent manner with many steps and as for overall yield? But that’s not so important. There are a few nice pieces of chemistry if one takes the time to read it. For example oxiranyl anion coupling. A 6 step sequence produced the ketone
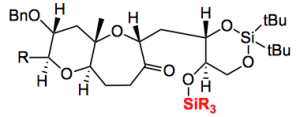
Concerning this sequence and the nature of the SiR3 goup the following statement can be read “the use of DEIPS as the protecting group was important, because the use of TBS decreased the yield of 14 owing to difficulties in its selective removal in the presence of a cyclic silylene protecting group“. This was discovered after 6 steps and the synthesis of a complex intermediate. Imagine going further and discovering this rather nasty effect. It’s bad enough after 6 steps, even worse after 20. Why choose DEIPS (which is presumably diethylisopropylsilyl)? Some fiendish chemical intuition, nope, time spent in the library.
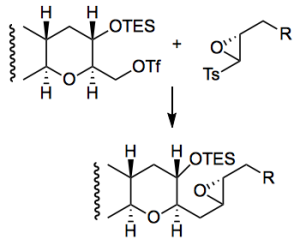
One of the oxiranyl anion couplings
Mix the triflate and the epoxide in THF/HMPA, cool to -100°C and add butyl lithium. Work-up after 30 minutes and isolate 86% yield of the coupled product. Interesting way to do this chemistry which presumably reduces the reactivity of the oxiranyl anion towards self condensation.
Lots of steps later one reads “completed the total synthesis of gymnocin-A (1) as an inseparable 91:9 trans and cis mixture. The 1H and 13C NMR spectra of the synthetic material were identical to those of natural gymnocin-A.” Now this is interesting because the reference supplied says the following “The trans and cis ratio of natural gymnocin-A was not reported“. Perhaps I’m missing something here? is the synthetic material identical to the natural product or not?
Something I would like to see is some comment on the amount of time it took to complete this synthesis. A long time, for sure. Now I know that people criticise such total syntheses. But they are a great exercise in practical organic chemistry, finding reagents, protecting group strategy purification, NMR spectroscopy and so on.
So 7 names and the boss on the paper reporting on a wonderful total synthesis of a fascinating class of natural products. Congratulations.
2,019 total views, no views today