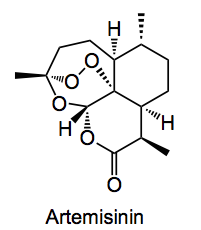
This week’s choice is a paper of the Sanofi groups from France, Italy and Germany, an international collaboration, lead by J. Turconi, discussing the process development that went into an industrial scale production of Artemisinin. I see that Chemjobber has already stolen some of my thunder and his piece on this process is definitely worth reading. So I shall try and highlight some other parts of the synthesis.
This is the anti-malarial treatment. Artemisia annua has been used by Chinese herbalists for more than two thousand years in the treatment of many illnesses, such as skin diseases and malaria. The earliest record of its use dates back to 200 BC. This compound has been the subject of many synthetic studies as for a long time it’s only source was the extraction from the plant leaves, this and other factors lead to an unreliable supply of material.
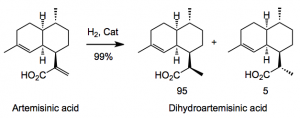
Obviously the peroxide moiety is mainly responsible for the biological activity, generating radical species in the presence of iron. This being the case it means that any chemical synthesis should install this group as late as possible in any given synthetic route. An interesting method was recently described by Seeberger employing a continuous flow system. Sanofi decided to employ a synthesis closely related to the biosynthetic route. The first challenge faced was the diastereoselective hydrogenation of the acrylate in artemisinin acid to produce the di-hydro system.
Lots of catalysts can achieve similar results. But what is important here is the cost of the catalyst and the ration of substrate to catalyst. Optimisation of the latter keeps the costs down and the catalyst load as low as possible. A screening by the “in house” discovered a useful catalyst: RuCl2[(R)-DTBM-Segphos](DMF)n at 22 bar of hydrogen with substrate/catalyst ratios of 8000/1 gives complete hydrogenation within a few hours providing a 99% yield of the two diastereoisomers after a simple work-up. This shows the added value of having your own catalyst group as part of the company, unlike others, I could mention, that decided to outsource that sort of mundane work (sarcasm here).
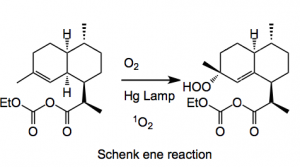
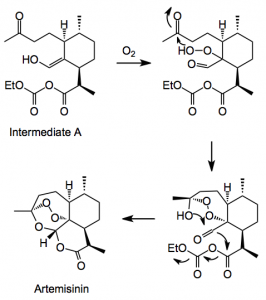
Now the photo-oxidation was investigated. The employed a Schenk ene reaction between singlet oxygen and a C=C followed by a Hock cleavage and oxygenation and cyclisation. Now this Hock cleavage is a reaction I now nothing about and the authors of this paper kindly provide a mechanistic rational for the observed products; firstly the ene reaction:
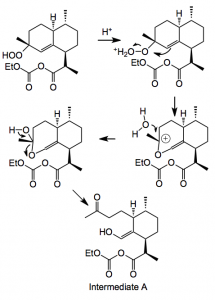
This reaction uses TFA and dichloromethane at -10°C and proceeded in high yield. Now comes the Hock cleavage reaction. I will reproduce the version found in the publication:
Step 1:
This delivers intermediate A which then adds another oxygen and eventually ring closes to give Artemisinin.
The overall yield for this step is 55% of isolated artemisinin. The photosensitzer used was tetraphenylporphyrin. The reaction was carried out in dichloromethane which cad be re-cycled. One should also note that Shenck ene hydroperoxide undergoes an exothermic decomposition at 15°C making the choice of reaction conditions very difficult. The following factors were considered for the photochemical process “We selected a semibatch mode concept with a recirculation loop, which is possible as artemisinin is very stable under the reaction conditions and it offers optimal conditions versus energy consumption and the transformation rate. In addition to the other chemical engineering challenges, critical parameters included construction materials that minimise the loss of light (optimising the quantum photonic yield) and the choice of a lamp with the optimal spectral distribution of emitted spectrum (medium- pressure mercury/gallium lamp). The unit must also ensure a good turbulence of the recirculating fluid and allow a good gas−liquid transfer, while maintaining the internal temperature at −10 °C. “
Also problematic is artemisinin itself, as one would expect it demonstrates an onset temperature of 160°C releasing 840 j/G, which is not an insignificant amount of energy! This and various other problems were solved and they ran a first photo-batch on a 50 kg scale. Production equipment was then installed and currently they can produce some 35 tons in 2013 and an expected 60 tons this year. The average batch size is 370 kg of isolated product. This is a tremendous achievement especially given the difficulties of photochemical reactions and the safe handling of peroxide intermediates and products.
So there we have it the final chapter in a long story in the production of this valuable compound. Congratulations to the team at Sanofi on a great job.
3,275 total views, no views today