This week’s choice comes from Natural Product Reports. Entitled Natural product synthesis in the age of scalability it was written by Christian A. Kuttruff, Martin D. Eastgate and Phil S. Baran. The Baran labs are, of course, well known for their outstanding natural product synthesis, as is the entire Scripps facility.
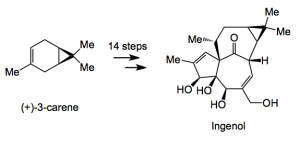
In this latest contribution the authors discuss the synthesis of selected natural products on a scale of 1 gram and above. This, for an academic laboratory, is a large undertaking as most are not really equipped to deal with such quantities, however it can be achieved as demonstrated by the Baran group’s recent synthesis of (+)-ingenol in 14 steps from (+)-3-carene.
The key reaction in this synthesis is a Pauson-Khand cyclisation using a rhodium catalyst in the presence of carbon monoxide and delivers an advanced intermediate, containing all the framework carbon atoms of ingenol, in 72% yield. This is certainly very elegant chemistry to reach such a complex structure, however, there is a way to go before it could be carried out on an industrial scale and as the authors say “the final few oxidation steps are quite limiting with respect to scalability and efforts are being made to address these issues”. However, this chemistry allows the provision of material for deeper biological evaluation and the route itself offers many opportunities for analogue synthesis.
The use of natural products as pharmaceuticals is somewhat hampered by their scarcity, not to mention their structural complexity. A typical example is artemisinin, which is a sesquiterpene lactone peroxide isolated from the the ariel roots of a shrub and is used an an effective anti-malarial.
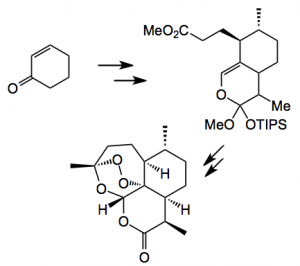
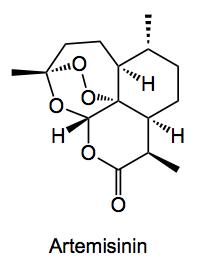 The world’s demand for this compound probably exceeds >150 tons per annum, causing supply a big problem. Fortunately this has been partially solved by a combination of bio- and organic synthesis. Several groups continue to work on various routes to this compound, most recently a continuous flow process has been published which utilises a photo-oxidation of a waste product from the plant extraction. Baran highlights a new route starting from cyclohexenone developed by Professor Cook at the University of Indiana.
The world’s demand for this compound probably exceeds >150 tons per annum, causing supply a big problem. Fortunately this has been partially solved by a combination of bio- and organic synthesis. Several groups continue to work on various routes to this compound, most recently a continuous flow process has been published which utilises a photo-oxidation of a waste product from the plant extraction. Baran highlights a new route starting from cyclohexenone developed by Professor Cook at the University of Indiana.
Thus in 7 steps this synthesis enables the construction of a complex molecule from simple cheap starting materials, normally not an easy task.
The Baran review cites many more interesting examples and can be recommended as an excellent read containing lots of useful information for those working in this area. A look at the Baran labs blog is also worthwhile, there is a link on this page. Enjoy.
9,352 total views, no views today