Secondment, what a nice word. After getting everything sorted out I reported for work on the 1st of March in one of the buildings of pharma. research on the Hq site, which was spread around several locations. There I met my new boss, a very nice gentleman who remains my friend to this day. He took me round the section and made the introductions. Lots of faces and names to remember. I was in the department dealing with the central nervous system, who were all busy synthesising and testing compounds with anti-depressant activity, anxiolytics, nootropics and the like. In our group we were interested in the GABA system of inhibitory neurotransmitters especially the recently discovered GABA-b receptor antagonists. It turned out that several of our phosphinate/phosphonate analogues of GABA and the commercial compound Baclofen (an agonist) were antagonists at the receptor. So why is this of any importance? Well antagonists block the receptor so the natural ligand cannot bind, in this case GABA, this results in increased GABA levels, and as it is an inhibitory neurotransmitter one may expect to see a variety of behavioural changes and even suppression of epilepsy.
So my job was to synthesise analogues of one of the first antagonists, and obtain an SAR series and of course more potent compounds, in vivo and in vitro. Also I had to examine the agonist series to find more than the two we had.
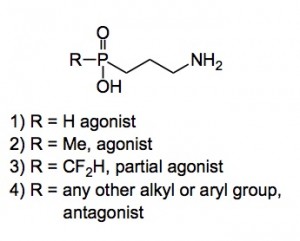 All this work has been published and you can see from them that the antagonist series was wide open, but the agonist series consisted of two main members. Compound 1 actually had a higher affinity for the receptor than the natural ligand by a factor of 15! I’ll spare you all the gory details of making methyl, ethyl, futile analogues of these compounds. In fact we, or rather I did not find anything of any use, well apart from two compounds
All this work has been published and you can see from them that the antagonist series was wide open, but the agonist series consisted of two main members. Compound 1 actually had a higher affinity for the receptor than the natural ligand by a factor of 15! I’ll spare you all the gory details of making methyl, ethyl, futile analogues of these compounds. In fact we, or rather I did not find anything of any use, well apart from two compounds
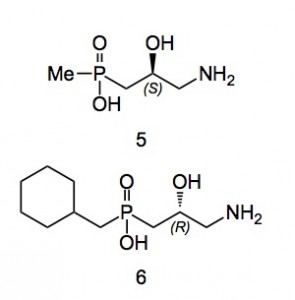 Compound 5 is an agonist with a reasonable receptor binding and is a muscle relaxant, compound 6 is an antagonist with strong anti-depressant properties, both are orally active.
Compound 5 is an agonist with a reasonable receptor binding and is a muscle relaxant, compound 6 is an antagonist with strong anti-depressant properties, both are orally active.
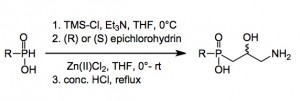
Now the preparation of these compounds has some lively chemistry attached to it. Both start out from the corresponding P-H compounds, after silylation they are combined with epichlorohydrin and a catalytic amount of anhydrous zinc(II) chloride to give the chlorohydrin. Further treatment with a nitrogen nucleophile and acid hydrolysis gives the final compounds.
Now the reaction with epichlorohydrin can be interesting. The kilo-lab was right next door to my lab, in fact right behind the wall at which I stared. One day I noticed something green along the windowsill which on closer examination turned out to be the arial root of a philodendron which had bored itself through the wall, says a lot for the building materials. Anyway on giving it a tug all the alarms went off in the building and smoke appeared in the corridor and from the windows of the kilo-lab. Firemen started to appear, as well as others in gas masks. I noticed this as I was evacuating.
Fortunately no-one was injured during this root tugging indecent. Later speaking to the kilo-lab man I found out what happened, we had had a runaway (reaction). The reaction mass had ejected itself up and out of the 20 litre reactor and the heat had generated some phosphine(s) which of course ignited causing the automatic CO2 system to go off. The smoke was triethylamine hydrochloride blown out before he could get the fume hood door right down. In spite of this mishap 97% of the reaction mass was recovered and I eventually received 90% pure product. I should also add that the plant survived and there was little damage to the fume hood.
More about phosphines in the next part.
2,218 total views, 2 views today