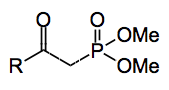
This week’s offering comes from the Paris labs of Cossy and tells us about the synthesis of β-ketophosphonates, useful reagents for the Horner-Emmons reaction.
These compounds are prepared by a couple of methods, one of which is the lithiation of methyl dimethylphosphonate and reaction with an appropriate electrophile. The problem with the lithium anion is its liking to undergo self-condensation or even better to transfer a methyl group giving the phosphonic acid monomethyl esters. This results in vast excesses needed to obtain the desired reaction. I can testify to that!
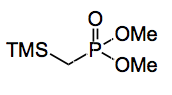
To get round these problems our intrepid investigators decided to use the dimethyl (trimethylsilyl)methylphosphonate:
This you synthesise from, wait for it, the lithium anion of dimethyl methylphosphonate in 66% yield! So the initial problems associated with the lithium anion have not really gone away, just moved towards the front of any synthesis.
To make the β-ketophosphonate you just throw in a suitable ester. But which ester? One where the ester part is a good leaving group. The pentafluorophenyl ester being the best (99% yield). So a whole range of pentafluorophenyl esters were prepared containing a variety of functional groups, epoxide, acetylene, lactones, bromides and so on. The two methylphosphonate anions were then compared in their reactivity towards the esters. Scanning the table in the paper does show that generally the silylated compound produces higher yields that the non-silylated compound. Where there is not a good leaving group on the ester e.g. acetate, the yields are very similar.
So is it really worth the trouble to make such an ester of your compound just to get 10-15 % higher yield? Well looking at the yields of the ester formation and combining those with the 66% of the silylated compound, probably not. Dimethyl methylphosphonate is cheap enough and with a bit of experimentation you can probably avoid the problems mentioned above. I did something very similar, several, years ago, with alkyl substituted methylphosphinates and Boc-glycine methyl ester, following a method originally published by Merck chemists for phosphonates (who are not cited in this paper). The phosphinate reactions worked, producing product in around 85% yield. I also did the phosphonates as well, before you all comment.
So unless you have a special case this method is not worth the hassle.
2,094 total views, 1 views today