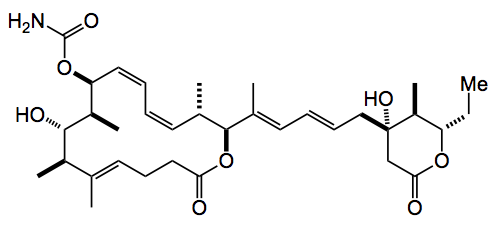
This one just didn’t get to me in time so I thought I’d slip it it at the start of the week, so if you are all lucky you will get two this time. It’s the total synthesis of the marine macrolide (-)-Leiodermatolide as devised by Paterson, Ng, Williams, Millican and Dalby, all at the University of Cambridge, UK.
This is a typical Paterson synthesis, full of stereochemistry, metal couplings and adol reactions. In a convergent route it was synthesised in 3.2% overall yield in 23 steps, which presumably refers to the longest linear sequence? Anyway employing the usual disconnections Ian managed to carry out a Stille coupling, a Heck coupling, a Suzuki coupling, and 4 stereoselective aldol reactions, not to mention the macrolactonisation and the formation of the carbamic acid.
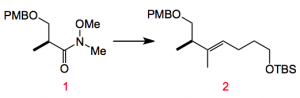
Starting from various Roche ester derivatives the complex stereochemistry is quickly incorporated along with the desired C=C stereochemistry. For example from the Weinreb amide 1, 3 steps provides the olefin 2 in about 70% yield:
Reagents: 1) TBSO(CH2)4MgBr, 2) LiHMDS, ArNTf2, 3) (MeBO)3, [Pd(PPh3)]4. (PMB = para-Methoxybenzyl)
The kinetic enolate of the intermediate ketone is converted into a 20:1, or better, mixture of the Z/E vinyl triflate by quenching with the Comins reagent. A Suzuki coupling finally delivered the olefin. The Comins reagent has the following structure:
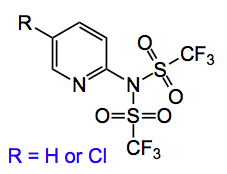 and is an excellent way of producing vinyl triflates just ripe for coupling reactions.
and is an excellent way of producing vinyl triflates just ripe for coupling reactions.
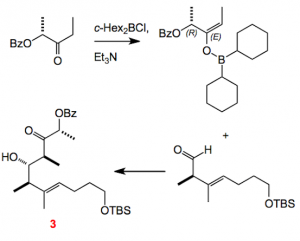
Turning now to the heart of the synthesis, the boron mediated aldol reaction. Over the years the Paterson group has perfected this reaction so that one can carry it out secure in the knowledge that you have almost complete enantio- and diastereoselectivity. Moreover the transfer of chiral information from the original substrate to the product can take place over a “long distance” enabling 1,6-stereo-induction, if required. An example from this synthesis will suffice as an illustration, for more information I refer you to the Group’s web site where there is a compilation of literature.
This matched anti-selective aldol reaction proceeds via generation of the E-enolate in 96% yield with a dr of > 20:1. I always find these reactions to be truly amazing, and the Cambridge group has exploited this over the years generating some remarkable total synthesis.
The comments above are only a fraction of the work presented in this paper and I recommend this publication to everyone as a lesson in stereochemical control as well as functional group manipulation. The group are to be congratulated on the success of this venture. May they continue to produce such elegant work for our enjoyment in the future.
5,906 total views, 2 views today