I recently wrote about a particular reaction in the synthesis of our 3d development compound. All the clinical material had been consumed and the boss decided that we should make more, 10 times more, still using the original route for reasons I will elaborate upon later. I decided that we were not going to do much more process research but just slog through the synthesis as it was. Little did I know.
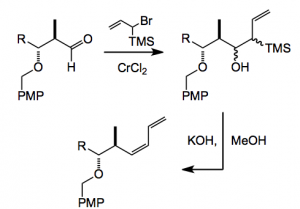
One of the reactions installed the cis-diene using a Nozaki-Hiyama-Kishi reaction, followed by a Peterson olefination:
PMP = para-methoxyphenyl.
Here the quality of chromous chloride is critical for the success of this reaction and once again this has not been adequately defined. There are suggestions that catalytic quantities of nickel (II) or palladium (II) may be required for an efficient coupling process. I did not examine the nickel (II) content of our chromous chloride. Indeed this may be a reason for the extreme variability observed during the large scale campaign which was in direct contrast to the previous campaign, where all the batches went to completion, without problems, in 2 hours at room temperature. Each of the three batch reactions behaved differently, the first was not complete after 2 hours and required more (20%) CrCl2 to be added with stirring for 18 hours at RT before it was complete. Prior to the second batch I made sure that the reaction started by taking a small sample and running it in the lab. It behaved as I expected. However, the large scale reaction did not budge one inch. I noticed that the samples taken from the reactor for reaction monitoring had all proceeded rapidly to completion. I surmised that this may have been an effect due to the contact of the sample with air (oxygen). To solve this I could have taken 150,000 samples and let them react but it was simpler to introduce air into the reaction vessel, a procedure that is fraught with hazard but I did it anyway. Lo and behold the reaction went to completion after a further 18 hour period. The third batch, however, when run under the conditions used for the second batch did not move at all. As a last resort it was warmed to 40°C and obliged us by going to completion within 2 hours! I might add at this point that all the reagents and starting materials were tested before starting the large scale batches, no problems were observed, all reactions going to completion within the 2 hour period.
So you may ask why I did not add come nickel(II) to get the thing going? Well we had an agreement with the QA department which said as long as we did not change the synthesis we could avoid re-doing the tox. study (which consumes lots of valuable material). And, we could not simply add a nickel(II) source, this would have to been ordered, analysed and released before we could have thrown a catalytic amount into the mix. Here is the crux of the issue, QA ! This project exploded the system simply because this level of complexity had never before been experienced both in terms of the chemistry and the SOPs used throughout the company.
To understand this you must realise that during “normal process research” you have adequate supplies of material and intermediates for all the optimisation work that goes on. But, in this case I didn’t have that luxury, towards the end of the route I was doing optimisation on an HPLC scale and going from there to the actual process, a scale-up of several 10s of thousands. QA demanded analytical specifications for each product. So not really knowing what to expect I set the specs. to be quite wide, giving us some room for the unexpected. This of course was rejected by QA who, especially in the GMP part of the synthesis, wanted tight analytical specifications. Eventually we compromised but not until a lot of effort had been expended.
The boss in the pilot plant also had a hard time with the chemistry. Many reactions were done at -78°C, for stereochemical control, however it took some convincing him that this low temperature was not a safety issue. A lot of the risk assessment processes in place at that time were not really designed with this chemistry in mind. For example, the cleavage go the Evans oxazolidinone with LiOH/H2O2/H2O, he refused to allow in his plant because of his issues with THF peroxides. Now this concern is not without justification, but in this case was not a hazard. Still we had to unload the reaction mixture, before work up, and transport it to a different location, do the work up and transport it back again. What a waste of time. Eventually the solvent was changed to methanol, which meant that 90% of the peroxide disappeared up the chute as oxygen and we used enormous quantities of nitrogen to keep the oxygen level below the explosion limit. Another problem was the in-silico tox. evaluation. I suggested that they evaluate the last 5 steps, what happened, the whole synthesis was evaluated. This of, course, raised lots of flags, and they produced a rather long report, causing unnecessary concern amongst the admin population. Mainly due to the reagents, because 99% of the intermediates had protecting groups stuck on they didn’t show up, so in effect the entire exercise was a waste of time. Solvents were also an issue. Someone asked me to supply them with a list of solvents used and the quantities involved, well that person is still waiting.
So this “new fangled” chemistry caused, not only chemical problems but administrative problems. The latter were often harder to overcome than the chemistry. Looking back on that synthesis, I believe we produced at least 10-20Kg of paper/gram of final drug substance. The standing joke was: In 1000 years time when the archeologists excavated the site, they would have concluded that there used to be a paper factory there.
More to come.
2,482 total views, 1 views today