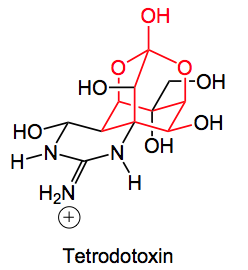
This week’s choice comes from the group of Alonso at the University of Santiago de Compostela in the place of the same name in Spain. It deals with the synthesis of a fragment, the dioxaadamantane core of tetrodotoxin shown in red.
As we all know this class of compounds are potent neurotoxins tetrodotoxin being found in the fugu fish. For further information on this series of molecules I can refer you to a excellent recent review by Du Bois etal which discusses the biology and synthetic chemistry of saxitoxin.
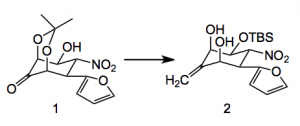
Alonso and his group have already been active in this area and have reported a formal total synthesis of tetrodotoxin. This current paper represents a new approach to the construction of the dioxaadamantane part of the molecule starting from the furan derivative 1, OH protection, Wittig olefination and acetonide cleavage gave compound 2 in 50% yield.
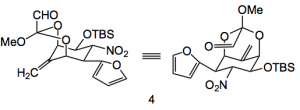
The orthoester was now formed with 2,2,2-trimethoxyethyl benzoate. Hydrolysis to the alcohol and Swern oxidation provided the key aldehyde intermediate 4 in high yield.
The molecule is now set for the key Henry reaction to complete the synthesis of the adamantane core. Using sodium hydride and water closed the ring to give compound 5. Now water in these reactions is usually fatal however, in this case it was necessary. The authors suggest that “Preliminary density functional theory (DFT) calculations revealed a lowering of the energy barrier for the nitroaldol addition when one molecule of water was incorporated in the calculation.20 The interaction of water with the carbonyl and furyl groups would facilitate the reaction while appropriately orienting the carbonyl oxygen away from the OTBS group. This would also explain the formation of the β-hydroxy nitro derivative as the single (desired) stereoisomer shown, which was isolated with an overall yield of 42% for the last three steps.”
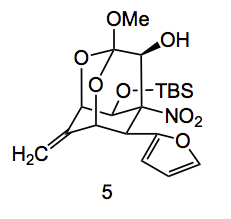 The authors then added the functionality required for tetrodotoxin in several steps including unmasking of the latent dimethylacetal groups present in the furan by RuCl3/NaIO4 cleavage to the carboxylic acid followed by reduction and IBX oxidation and finally acetalisation.
The authors then added the functionality required for tetrodotoxin in several steps including unmasking of the latent dimethylacetal groups present in the furan by RuCl3/NaIO4 cleavage to the carboxylic acid followed by reduction and IBX oxidation and finally acetalisation.
As is the world of chemistry the conversion to tetrodotoxin itself has been hindered by the stability of the methyl orthoester. All attempts to remove the methyl group have been unsuccessful. However I am confident that they will be able to apply this strategy to make the toxin and look forward to reading about it. Very nice approach, congratulations.
2,575 total views, 1 views today