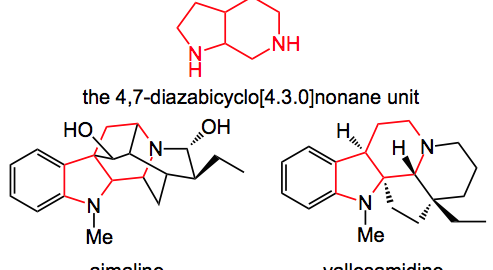
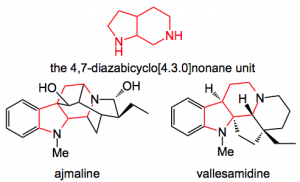
This week’s offering appeared in Angewandte Chemie and was presented by Prof. Waldmann and discusses his results with the 1,3-dipolar cycloaddition leading to the 4,7-diazabicyclo[4.3.0]nonane unit which is an integral part of various alkaloids such as ajmaline, vallesamidine and so on.
The process under discussion is of course well described in the literature and I have commented on them in the past. Waldmann employs a catalytic enantioselective intramolecular cycloaddition to construct the bicyclic unit shown above. This is all done in one pot and gives good yields and very high ee%s.
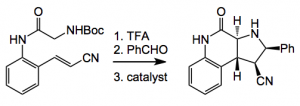
As usual in these cases the cycloaddition conditions were optimised as were the catalysts necessary for the generation of high ee%. It was found that in order to produce high enantioselectivity the isolation of the intermediate Schiff’s base was required. For example:
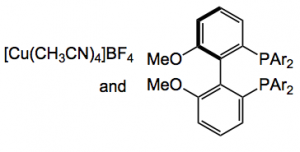
The yield of this reaction is 80% and the %ee 98%, it does not work if the Schiff’s base is not isolated. The catalyst is 5 mol% of a copper based system:
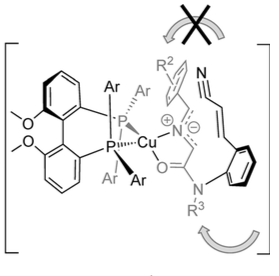
The high enantioselectivity is explained by the following transition state:
From the authors:”Owing to the steric requirements of the intramolecular cycloaddition, in the reactive conformation, the amide must be cis-configured, such that the olefin can approach the dipole. The dipolarophile approaches the dipole in an endo transition state to minimize steric inter- actions and strain. The attack of the electron-poor olefin preferably occurs from the front. Facial differentiation is achieved by means of the alkoxy-substituted aryl substituents on the phosphines (Figure 2). Upon attack from the back of the dipole, the cyanovinyl group would face unfavorable interactions with the aryl group pointing towards the back. Owing to the ligand stereochemistry established by the atropisomeric bis(phosphine), the second aryl group of this phosphine points downwards such that in the shown orientation of the dipole, steric interactions are minimized”.
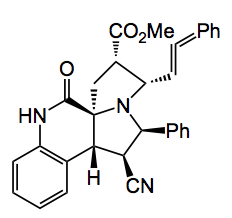
Treatment of the cycloaddition products with an aldehyde followed by deprotonation produces another azomethine ylide which cycloadds to a dienophile. So if you do it correctly a tandem cycloaddition product arises. Reaction of the product from above with cinnamaldehyde and methyl acrylate give the following compound in 60% yield:
A final quote”The tandem sequence displayed a very appreciable substrate scope, and the substituents in both the first and the second dipolarophile could be widely varied. Notably, the introduction of disubstituted olefins in the second cycloaddition step in a one-pot procedure yielded annulated pyrrolizidines with seven contiguous stereogenic centers, including a quaternary carbon atom.”
So here is a really good demonstration of the azomethine ylide cycloaddition delivering complex molecules in few steps in high yield and enantioselectivity. I think this must be one of my favourite reactions, probably because I also had some success with it!
2,597 total views, 3 views today