Ever worried about traces of water spoiling your reaction or product, well your worries could be over with the publication of an Angewandte ASAP by Prof. Manos and colleagues discussing the development of a metal organic framework (MOF) luminescent sensor for the detection of traces of water in organic solvents. Karl-Fischer must really be starting to worry now.
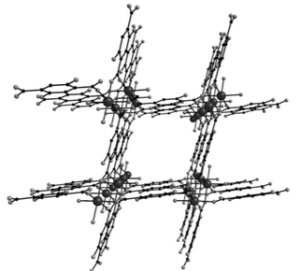
Its structure is [Mg(H4dhtp)(H2O)2] . DMAc where dhtp = 2,5-dihydroxy terphthalic acid and DMAc = N,N-dimethylacetamide. It is prepared by simply reacting magnesium diacetate tetrahydrate with H4dhtp in DMAc/H2O (9:1 v/v) at 120°C. It forms paltelike crystals and belongs to the monoclinic space group C2/c and looks like this:
It “features one crystallographically unique Mg2+, which adopts octahedral coordination geometry and is linked with four carboxylate oxygen atoms from the H2dhtp2- ligands and two terminal water ligands. Each Mg atom is connected to two neighboring Mg atoms by four η1:η1:μ2-COO-, thus forming a chain (rod) of linked MgO6 octahedra running parallel to the c axis (Figure 1 a). The chains are interconnected through the H2dhtp2- linkers to form a 3D framework (Figure 1 b) which displays a solvent-accessible volume of about 45 % and size of pores approximately 6Å”.
Shining UV light on the MOF-DMAc complex gives an intense turquoise colour. The MOF itself shows a yellow emission and MOF-water gives a yellow-green fluorescence. The addition of aliquots of water into a suspension of the MOF in dry THF results in an overall enhancement of fluorescence intensity with a red-shift of the fluorescence maximum from about λ = 455 to 530 nm. “One more advantage of MOF as a moisture sensor in THF is that it shows two types of signal transduction upon analyte (water) uptake: 1) an overall enhancement of luminescence and 2) a shift in the emission maximum. Thus the MOF offers two means of analyte detection thereby increasing specificity and eliminating possible sources of misinterpretations“. This also works for water in ethanol and acetonitrile.
This is obviously a very brief description of what exactly is going on in this system and the authors go into great detail to explain the mechanistic background, which I admit I don’t fully understand. But here we have a very useful method, especially for analytical labs who do lots of water determinations. I know the titration methodology is automated these days however I’m sure this method is robust and could be quickly validated and qualified for GMP purposes. Even in a normal lab environment it should be a useful tool.
So get making MOFs! Judging by this paper you can probably make a MOF to do this for most solvents? Which would be very useful and a nice compliment to the current GC methodology.
2,047 total views, 3 views today