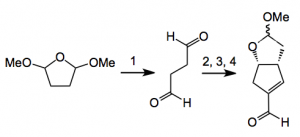
This week a somewhat strangely entitled but interesting paper from the Aggarwal group at the university of Bristol, UK. The title is “Synthesis of Prostaglandin Analogues, Latanoprost and Bimatoprost, Using Organocatalysis via a Key Bicyclic Enal Intermediate”. The only organocatalytic step in the multi-step route to these prostaglandin analogues is the second one:-
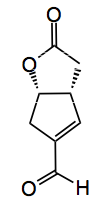
Reagents: 1), water 75°C; 2) 2 mol% (S)-proline, 2-methylTHF, rt, 20 hr; 3) 2 mol% [Bn2NH2][OCOCF3], THF, rt, 20 hr; 4), MeOH, amberlyst 15, DCM, rt. Yield for these steps 14% from the succinaldehyde intermediate. Also prepared was the lactone by oxidation in air catalysed by a copper triflate/TEMPO system in 93% yield from the lactol.
Now the authors hope that this chemistry will form the basis of a viable manufacturing process. This is a worthwhile aim however I don’t think it will make it. A look at the supplementary information suggests why. A 14% yield is a killer in any synthesis be it at the start, middle or end. If you are proceeding towards clinical supply you are going to require large amounts of either the lactol or lactone. The very low yield means very large quantities of materials must be used.
The synthesis, as described, from 2.5-dimethoxyTHF involves some complicated distillation manoeuvres combined with azeotropic removal of water with temperatures ranging from 75°C to 115°C followed by a reduced pressure distillation at 70°C/120 mbar to 100 mbar, then a Dean-Stark at 95°C normal pressure (I assume, because it is not stated). All this to achieve a solvent change from water to 2-MeTHF. Yields between 61-87% of succinaldehyde are claimed as judged by NMR!! Something tells me that the aldehyde and perhaps the product as well are not very stable under these conditions. An examination of their thermal properties by DSC may have provided more information for the authors and the reader.
For the proline catalysed aldol reaction a 2M solution in 2-MeTHF is used, again quite dilute. For the second aldol THF is added followed by the catalyst. Why THF? Why not 2-MeTHF, or THF from the start? This makes no sense. From the authors “By using our original optimized conditions, enal can be isolated in 14% yield, but occasionally, valuable material is lost upon workup. To reduce these losses, we undertook further optimisation studies and discovered that adding charcoal to the reaction mixture at the end of the process followed by stirring, filtration, and purification led to more efficient removal of oligomeric material without loss of significant quantities of the valuable enal. We now have a more reliable process, which consistently provides our key enal intermediate in ca. 14% yield.” Now I noticed that they have patented this process, unfortunately I do not see the novelty here but never mind.
Adding active charcoal brings a whole set of new problems to any process. In this case they use very, very large amounts, 100g for about 60g of succinaldehyde. Using charcoal is messy and the cleaning of reactors is a nightmare. Not to mention; how long do you treat the reaction mixture with it to obtain the optimal removal of unwanted side products? It was not mentioned in the detailed experimental procedures so we are left to guess. What about the particle size, ease of filtration, and I could go on and on. Better to leave it out and find a set of reaction conditions which does not need charcoal.
The work-up and purification requires chromatography to give the lactol in reasonable purity, as an oil. Chromatography required 600g of silica, not stated was how much crude material was placed on the column. The methylacetal also required chromatography, 300g of silica, again the amount of material chromatographed was not reported. If you go from weight of the the starting material, 4 grams, then the ratio of reaction mixture to silica is about 1:100. Imagine the size of the column if you have 100 kg of reaction mixture! I won’t even comment on the Cu catalysed reaction.
While this chemistry undoubtable works as reported I do not agree that this is even a basis for a viable, reliable, reproducible process. Perhaps the authors should consult a process chemist.
Assuming that the problem is the stability of succinaldehyde the sequence could be made much better and more efficient using some of the flow chemistry methodology that is appearing more and more in the literature, especially those designed to handle reactive intermediates and products and do not use charcoal.
8,615 total views, 2 views today