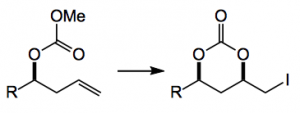
This week Gaunt published an approach to 1,3-syn diols (useful building blocks in natural product synthesis) using an iodolactonisation approach and a copper catalyst. Based on an apparently under utilised reaction, the synthesis of a cyclic carbonate from a homoallylic carbonate:
Of course the alkyl iodide can be used as a useful synthon for further transformations.
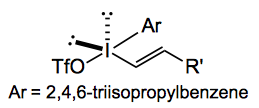
Gaunt has discovered that the iodolactonisation reaction can be done using “diaryliodonium reagents in combination with copper salts as a catalytically generated source of aromatic electrophile equivalent.” The iodine source has the following interesting structure:
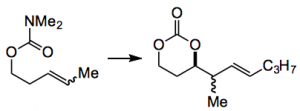
Using 2 equivalents of the above and the toluene complex of Cu(I) triflate the cyclic carbonate was formed in low yield, <20%. You can just imagine the comments, especially as the dr was reasonable. The situation was resolved when they used the dimethylamino carbamate instead of the methoxy mixed carbonate. This gave the desired product in 96% yield!
R groups are well tolerated, varying from H to alkyl, haloalkyl, silyloxy methyl, benzyloxymethyl, all giving the cyclic carbonate in medium to high yield with good dr. An internal alkene also reacts well:
the (Z) alkene gives the syn/anti product in a dr of 1:6 and the (E) alkene 15:1 syn/anti, both in high yield.
The R’ salt substituent can also be made into something more useful than an n-propyl group. For example, propenyl, vinyl, acrylate, all of which allow elaboration into more interesting functionality. Yields of these are again reasonable with excellent dr.
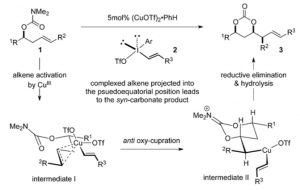
The mechanism, proposed by the authors is :
which was borne out by various observations. The reaction can be re-iterated multiple times by carbonate cleavage to the diol followed by conversion to the carbamate and renewed cyclisation thus extending the chain at both ends. An author’s summary “we have developed a new copper-catalyzed oxyalkenylation of homoallylic carbamates. The transformation is tolerant to a wide range of functional groups and provides ready access to a broad range of 1,3-carbonate products in very high yields and good selectivity. The cyclic carbonate products can be further manipulated into 1,3-diol derivatives which are amenable to further iterations of the reaction. Ongoing research is focused on elaborating on these studies by applying the methodology in natural product synthesis.”
So you can bet the natural product synthesis will be underway, perhaps even done? A very useful robust method here which I hope will se a lot of use.
1,916 total views, 1 views today