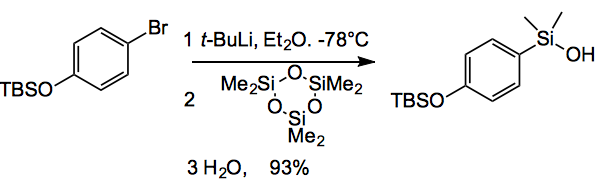This week another review with the provocative title “Why You Really Should Consider Using Palladium-Catalyzed Cross-Coupling of Silanols and Silanolates”. Now this appeared in OPRD and it was penned by Denmark.
So why should we consider these silicon-based cross coupling methods? Mainly two reasons, 1) “a 2011 toxicological report unveiled4the mutagenic properties of several boronic acids and esters. These results are further strengthened by a recent study that discloses the mutagenicity associated with a large number of boronic acids, esters, MIDA boronates, and potassium trifluoroborates, as well as their precursors, bis(pinacolato)diboron and bis-boronic acid. The newly assessed genotoxicity of boron compounds naturally poses some serious limitations to their applications in medicinal and process chemistry, both in terms of worker exposure hazards and more stringent standards of purity to avoid the persistence of genotoxic impurities”. and 2) most of the chemistry discussed was developed by him. Now the first is news to me and puts a different light on the traditional coupling methods. No-one wants genetoxic/mutagenic compounds in their mixtures especially with all the complications that are associated with this particular set of molecular properties.
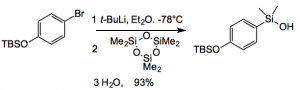
In any case silanol coupling is, of course, very similar to boronate coupling, with the usual set of Pd catalysts being employed. The point is making the silanols. This is very throughly covered by Denmark in this review. For example:
For less reactive or more unstable organometallics one uses dimethyldichlorosilane or dimethylchlorosilane. With subsequent conversion by hydrolysis or oxidation to the silanol. Alkyne hydrosilylation can also be employed as can Pd catalysed C-H silylation of arenes.
Cross-coupling is very much according to the standard methodology. Palladium catalysed reactions in the presence of TBAF is used for aryl iodides and vinyl iodides to couple silanols. Geometry of olefins is normally preserved. Triflates also work well. Aryl and heteroaryl-silanols are less reactive. The former require water (usually water of hydration) in order to prevent siloxane formation. The latter need de-protonation and the presence of 1 equivalent of Cu(I) which limits protodesilylation. Using Cu can be avoided if the deprotonation is stoichiometric forming the silanoates , otherwise quite normal Pd coupling reactions. Yields are normally good to excellent.
“The advantages of organosilanols as coupling partners are manifold in comparison to other silanes, including (1) ease of synthesis, (2) stability toward oxygen and moisture, (3) ease of purification, (4) diversity of synthetic methods for their preparation, and (5) functional group tolerance. An addi-tional advantage of significant synthetic importance is the ability to activate the cross-coupling of organosilanols with Brønsted bases, thus avoiding the incompatibilities associated with fluoride. Moreover, the conjugate bases of organo- silanols are stable, often free-flowing powders that are “self- activating” cross-coupling partners, i.e., they require no additional activators“. And the authors recognise that “One of the unique advantages (and, to some extent, also drawbacks) of silicon is the literally dozens of organosilane moieties that can participate in cross-coupling reactions, from the most inert trialkylsilanes to the highly reactive trichlor- osilanes. This diversity allows for precise tuning of the reactivity and functional compatibility of the donor for a specific cross- coupling reaction. Of course, this diversity also presents a challenge for the experimentalist to identify which of the many options would be optimal”.
So a useful alternative to the boron approach. Although, as this is published in OPRD I would have appreciated a bit more information as to the thermal properties of these silanols to assist in any selection. More about the “more unusual” catalysts would also have been useful. Also alternatives to some of the more “reactive” reagents might have been presented. Perhaps they were, at least referenced to, but I did not read all the referred publications.
A flow system would be a good solution to this aspect and some of the other potential problems. If this has been published then I apologise for not mentioning it. Something more for the tool-box here. Perhaps it needs a name? The Denmark Coupling springs to mind.
1,872 total views, 1 views today