This week’s ASAP presents us with chemoselective nitrile alkylation and comes from the Fleming group at Duquesne University, Pittsburgh. The method depends upon selective electrophile attack depending upon which atom the metal is coordinated to, the nitrile nitrogen or the nucleophilic carbon.
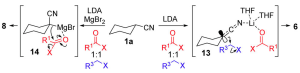
Cyclohexanecarbonitriles were used as the model system as either the preparation of C or N metallated is well described.
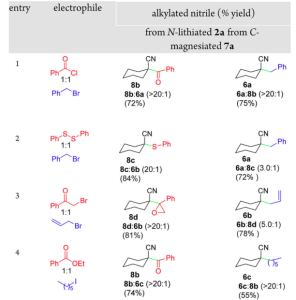
So when X = H and Y-LG is a 1:1 mixture of benzyl bromide and methyl cyanoformate with LDA as the base the product where Y = benzyl is exclusively obtained in 98% yield. When X = Br and Y-LG is the same mixture forming the Grignard reagent with iso-propylmagnesium chloride produces the product where Y = carboxymethyl in 70% yield. Other metals were used; the cuprate and the zincate they showed the same tendency as for the Grignard reagent suggesting that the chemoselectivity is determined by the metal coordination site. Experiments indicated a general preference of the magnesiated nitrile for a range of oxygenated electrophiles whereas the lithiated nitrile had a more limited preference for alkyl halides. For example the table shows some nice selectivity:
“The ability of the N- and C- metalated nitrile structures to direct the chemoselective alkylations suggests that associative electrophile interactions control the selectivity, a notion supported by the disruptive influence of LiCl. Addition of a mixture of electrophiles to a lithiated nitrile likely results in coordination of oxygen-containing electrophiles to the Lewis acidic lithium which serves to prevent alkylation by anchoring the electrophile remote from the nucleophilic carbon. Alkyl halides, being weaker Lewis bases, may be able to directly approach the nucleophilic carbon resulting in alkylation” A mechanistic rationale was also presented:
From the authors “In summary, chemoselective alkylations of N- and C-metalated nitriles allow preferential nucleophilic attack with different electrophiles. For comparable alkylations from the same nitrile precursor, N-lithiated nitriles prefer to react with alkyl halides, whereas C-magnesiated nitriles preferentially alkylate oxygenated electrophiles. Alkylations with bis-electrophiles allow selective attack at different electrophilic sites simply by judicious choice of the metal cation. The chemoselective alkylations of metalated nitriles offers the possibility of selective, late-stage alkylations of polyfunctional electrophiles for efficient syntheses and the creation of diverse natural-product like libraries.”
So a nice thorough study of an important system for nitrile alkylation.
2,064 total views, 1 views today