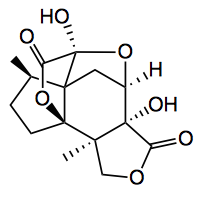
I read the recent Nature Chemistry paper by Shenvi with interest. Nice chemistry leading to (-)-jiadifenolide in 8 steps. Before I comment, I would like to say that the chemistry reported here is great stuff, interesting, relevant reactions, but be careful using the new “buzz words”.
Eight steps, I actually counted more, recognising the fact that chromatography and distillation are also “steps” in a synthesis and if ever this route were to be scaled to be a drug substance process these steps would have to be eliminated or kept and validated just as the chemical ones have to be. I suppose I’d better not mention the step requiring -100°C!
I wish academics would not make sweeping statements such as “the procedure proved capable of producing multi- gram quantities of xxx at reasonable batch concentrations (0.1 M)“. A 0.1M batch concentration is actually very dilute. The compound in question has a MWt. of 154 and produces product in 4 steps in 28% yield. The steps involve elemental bromine, triphenylphosphite, -78°C, chromatography; then tBuOK 18-crown-6 in hexane, distillation. This is followed up by ozonolysis and reductive work-up with dimethylsulfide and a chromatography. Finally a sealed tube reaction with molybdenum hexacarbonyl (note behind a blast shield) and repeated 6 times! Finally another column. So this sequence is actually 8 steps. Maybe an iron carbonyl stuck somewhere on a porphyrin would be better?
Imagine you are the poor post-doc that needs 50g of this compound for your further research. According to the supplementary information you can use conventional heating to get 56% yield. The sealed tube reactions were done using 0.176 g (6 times) or one reaction of 4 g with yields of 50% and 58% respectively to give a dark oil which was chromatographed. I ask, “what is the rest of the material?” There is no information in the paper and there damm well should be. This seems to me to be an accident waiting to happen.
So you want 100 kg of this starting material, 8 steps, chemical yield 28% requires around 400 kg, or 2,500 mole of the starting material. At a concentration of 0.1M this will use hundreds of thousands of liters of solvent. I won’t even go and mention the reagents. So my question is, why didn’t they use a flow chemistry system? This would be ideal for this application.
Now before you all shout that this is the realm of a development chemist, I agree. It is our job to optimise reactions, find new reagents to do the conversions, eliminate chromatography, etc. But one of the above steps is a distillation, is this really required? I once had to distill >1000 kg of a product, it took me 6 months day and night, using our apparatus (we didn’t have a bigger one) and that was optimised by a distillation expert. What happens if you are really unlucky and there is no way around, you are stuck with the original synthesis. I suppose you just have to run hundreds and hundreds of sealed tube reactions and hope none go bang. OR use a flow set-up.
So, dear academics, although you have made gram quantities of material in your lab does not mean that you have a real gram-scale route that everyone can carry out. Please avoid the Headline Caption “Gram Scale”.
4,699 total views, 1 views today