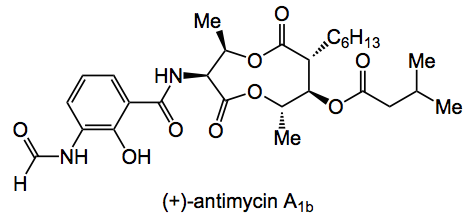
This week the total synthesis of (+)-antimycin A1b caught my eye. It was described by Batey and Janetzko from the University of Toronto and appeared in JOC:
The whole family of the antimycins shows a plethora of biological activity from anti-fungal to cardioprotection. As usual with such compounds there are a number of synthetic challenges to be dealt with. In this case, so we have the formation of the 9-membered bis-lactone ring, the stereotriad needs to be installed, not to mention building in the formylamido salicylate ring.
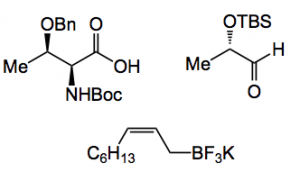
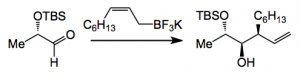
So following a normal sort of retro-synthetic approach they arrived at the precursors below, 2 from the chiral pool and an allyl boronate salt:
This boron reagent was chosen because previous work from the group suggested that the diastereoselective crotylation of α- and β-silyloxy aldehydes using (E)- and (Z)-crotyltrifluoroborate salts can be achieved under phase-transfer-catalyzed conditions. From the abstract of the previous work “Potassium allyl- and crotyltrifluoroborates undergo addition to aldehydes in biphasic media as well as water to provide the corresponding homoallylic alcohols in high yields (≥94%), excellent diastereoselectivity (dr ≥ 98:2), and without the necessity of any subsequent purification. The presence of a phase transfer catalyst (e.g., nBu4NI) significantly accelerates the rate of reaction, whereas added fluoride ion retards the reaction.“
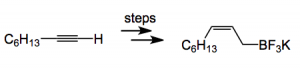
This boronate salt is stable and easy to use (demonstrated nicely by Molander and his group), in contrast to the more usual types of boron reagent which, as we all know, are somewhat touchy! The salt was prepared in several steps from the acetylene:
The “steps” represent a relatively long sequence involving metalation, trans metalation to a boron species which was then subjected to hydroboration and selective protodeboration. Eventually the potassium salt emerged. In all 9 steps, with an overall yield of 44%. This chemistry is claimed to be scalable, at least to gram scale. A quick look at the experimental section reveals a few problems on the 100 mmol scale: Lots of diethyl ether for one, lots of low temperature steps, lots of extremely air sensitive reagents (glove box), stirring problems, chromatography, exothermic reactions with “appreciable frothing” reactions with “vigorous gas evolution“! I think the authors need to re-define their definition of scalable.
This chemistry is not trivial and I certainly would not like to try this out. For one thing the use of diethyl ether needs to be examined, and a replacement found. The “appreciable frothing” must be kept under control as must the “vigorous gas evolution” not to mention the exothermic reactions. A lot of care needs to be taken with this chemistry to avoid any nasty incidents.
Continuing: the addition of the potassium salt to the aldehyde proceeded as expected to give the key intermediate containing the stereo-triad:
Using montmorillonite K10 clay as an acid catalyst (a method developed by Batey) the addition went in 90% yield in a two phase system to give the product with a d.r. of 94:6.
Jumping to the conclusion, antimycin was finally obtained in 19 steps with an overall yield of 18% (in the linear sequence). Interesting chemistry here, but not trivial to do due to the synthesis of the allyl potassium boronate reagent. If I was tasked with this chemistry I would certainly like to know much more about the chemistry leading up to this compound. As it is described it is sub-optimal and they should be really careful when scaling this up. However they survived to write the paper and that is always a good thing. Congratulations on a nice synthesis.
4,980 total views, no views today