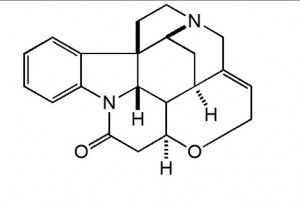
4aR,5aS,8aR,8a1S,15aS)-4a1,5,5a,7,8,8a1,15,15a-octahydro-2H-4,6-methanoindolo[3,2,1-ij]oxepino[2,3,4-de]pyrrolo[2,3-h]quinolin-14(4aH)-one
Imagine if Agatha Christie had to write that every time she had to mention the poison used in the murder, or if Hitchcock’s leading man had to vocalise it in the courtroom. Well they’d never get the book or the film down to a manageable size. It’s much easier to say strychnine!
From the early eighteen hundreds until the present day strychnine has been the subject of intense study. In a recent review in Angewandte Chemie International edition Professor Overmann and Dr. Cannon present the history of this fascinating compound in terms of total chemical synthesis, the title says it all, “Is there no end to the total synthesis of strychnine?” They are to be commended for presenting the complete history of one of the world’s most famous murder tools.
I won’t recount the total synthesis here, except to pick out a couple of salient highlights. My wish is to give the community a feeling for the effort, which went into the structure elucidation and total synthesis of this remarkable compound.
The statistics of strychnine research are truly impressive: In the middle ages people were aware of the properties of the ground nuts of Strychnos nux-vomica and physicians in Germany in the 16th and 17th centuries were also curious about this preparation. In 1818 the active ingredient was isolated and was reported to have a variety of pharmacological properties. However, its only real use was as a poison. Around 50mg is fatal for an adult. So if your average adult is 70kg, this makes a lethal dose of around 700ng/kg, which is toxic. It acts on the central nervous system binding at the glycine receptor chloride channel and causes convulsions and asphyxiation. There is no antidote.
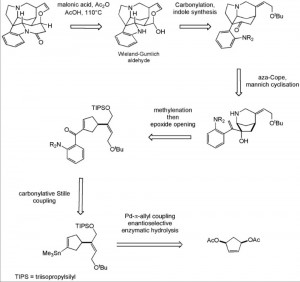
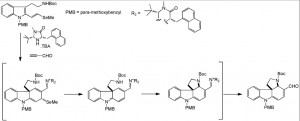
This was one tough nut to crack (pardon the pun), Robinson etal apparently published around 400 papers on the structure determination of strychnine, 400 papers, gracious they must have spent more time writing than experimenting. In those days it was all done by degradation and conversion of the fragments to a known set of compounds and then all put back together in a vast mental effort requiring a supreme knowledge of organic chemistry. Woodward finally solved the structure that was later confirmed by x-ray. Some 58 years ago he published the first total synthesis of racemic strychnine, only six years after the structure had been determined. Forty years after that the first enantioselective route was described by Overman etal. Here is his retro-synthetic pathway:
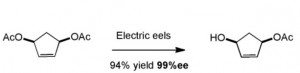
Above the arrows are the proposed synthetic steps. One of the interesting conversion here is the de-symmetrisiation of the diacetate using shocking conditions. Stirring this compound with electric eels, or better the acetylcholinesterase produced from the poor creatures, hydrolysed the AcO group on the left as shown:
One can imagine how the reaction vessel must have looked. Further highlights are the use of η3-allylpalladium alkylation and carbonylative cross-coupling reactions.
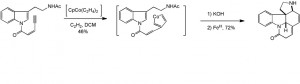
A cobalt catalysed [2+2+2] cycloaddition strategy was employed by Vollhard, in his route to racemic strychnine, to construct four of the six rings, in reasonable yield:
From here he had a further six steps to the final product.
Moving right up to date Macmillan published a route involving several interesting chemical reactions. He employed an enantioselective organocatalytic promoted sequence to produce an advanced intermediate in 87% yield with 97%ee via the pathway shown here (taken from the Overmann review).
So there is just a part of the more than 200-year-old strychnine story.
Here is an interesting table taken from the review somewhat abbreviated.
| Main Author | Year | Target | No. of Steps | Overall Yield (%) |
| Woodward | 1954 | (-)-isostrychnine |
29 |
0.0002 |
| Overman | 1993 | (-)-Wieland-Gumlich aldehyde |
25 |
3 |
| Rawal | 1994 | (±)-isostrychnine |
12 |
10 |
| Kuehne | 1998 | (-)-Wieland-Gumlich aldehyde |
21 |
4 |
| Vollhardt | 2000 | (±)-isostrychnine |
14 |
0.7 |
| Martin | 2001 | (±)-Wieland-Gumlich aldehyde |
16 |
1 |
| Fukuyama | 2004 | (-)-Wieland-Gumlich aldehyde |
25 |
1 |
| Reissig | 2010 | (±)-isostrychnine |
9 |
4 |
| Vanderwal | 2011 | (±)-Wieland-Gumlich aldehyde |
6 |
2-3 |
| MacMillan | 2011 | (-)-Wieland-Gumlich aldehyde |
12 |
7 |
Isostrychnine and the Wieland-Gumlich aldehyde are convertible to strychnine in one step in reasonable yield 28% and 80% respectively. For more detailed information please refer to the Overman essay and the references therein
2,969 total views, no views today