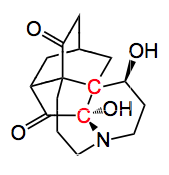
This week’s offering comes from the University of California at Irvine and was written by Sizemore and Rychnovsky. It deals with an Intramolecular Diels-Alder reaction approach to the core ring system of the isopalhinine lycopodium alkaloids. The structure of isophalhinine A is below.
The core bicyclic structure belongs to the isotwistanes. Which I recognised instantly! These chemists are certainly not afraid of a challenge as they wished to find a route that could allow access to this entire alkaloid family. Notwithstanding the bicyclic core, the molecule has several nasty stereochemical challenges to overcome, especially the two vicinal chiral quaternary centres (shown in red). Their solution to access a key fragment of the alkaloid structure demonstrates the power of the Diels-Alder reaction, especially the intramolecular version.
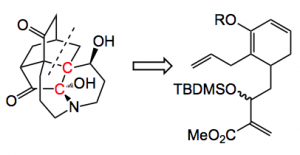
It took me a while to figure out the disconnection, before I read the next page and saw it:
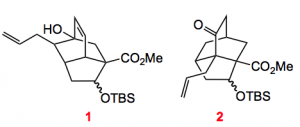
The enol ether is put together in 11 steps including an organocatalysed Morita-Baylis-Hillman reaction. Now the interesting thing is that depending upon which conditions were used to form the enol ether (R = TMS) the result of the intramolecular Diels-Alder reaction was different. Using TMStriflate/triethylamine in methylene chloride followed by heating to 180°C gave a 1.5:1 mixture of bicyclics 1 & 2.
Compound 1 was formed in 40% yield with a dr of 2:1, compound 2 in 28% yield with a dr of 1:1, all 4 compounds being separable. If the enol ether was formed with TMSCl/Et3N in DMF at 90°C then only a 1:1 mixture of compound 2 was formed. This is the desired isomer for the synthesis of the targeted alkaloid. However, compound 1 can also be utilised to synthesise alkaloids of a related family. The Rychnovsky group is currently trying to figure out the energetics of this Diels-Alder in order to explain this result, good luck!
All in all this paper is a wonderful demonstration of the power of synthetic organic chemistry. I wish the authors good luck in advancing this intermediate to the target, which we will read about in future publications.
4,308 total views, no views today