Here is another congested natural product, Sculponeatin N, whose synthesis was described by B. Moritz, D. J. Mack, L. Tong and R. J. Thomson from Northwestern University, USA.
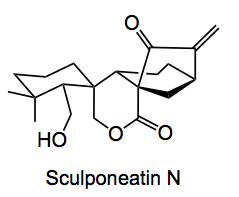 This one is a bit complex, 3 quaternary centers, two of which are chiral, not to mention the bicyclo[3.2.1]octane ring system. It was isolated from the extracts of plants of the Isodon genus found in China and used for traditional oriental medicine.
This one is a bit complex, 3 quaternary centers, two of which are chiral, not to mention the bicyclo[3.2.1]octane ring system. It was isolated from the extracts of plants of the Isodon genus found in China and used for traditional oriental medicine.
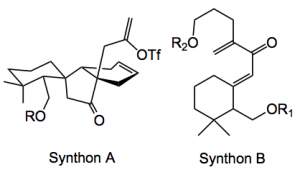
This structure cries out for a Diels-Alder reaction to construct the complex ring system, this gives synthon A as a retrosynthetic intermediate which can be accessed by a Nazarov cyclisation of synthon B.
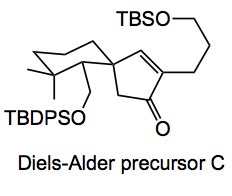
Previously, Thomson used a stereoselective Nazarov cyclisation followed by an oxidative ring expansion of the resulting cyclopentanone to prepare the C10 quaternary stereocenter and lactone of a related seco-terpene. In the current synthesis the Diels-Alder precursor C was obtained after 6 synthetic steps, including Grignard addition, Peterson olefination, alkylation and Nazarov cyclisation.
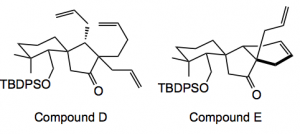
Unfortunately the cyclisation reaction did not work. Probably a variety of reasons for this, but I bet it was frustrating for the team. The desired ring system, compound E, was eventually obtained by preparing compound D and exposing it to the Grubs II catalyst:
The terminal C=C in E is readily converted to the enol triflate via the corresponding methyl ketone by Wacker oxidation and reaction with the Comins reagent (which has already been presented on this site).
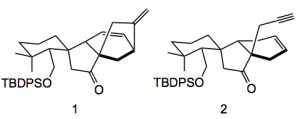
Remaining was the cyclisation to produce the bicyclo[3.2.1]octane. A normal Heck coupling produced the six-membered ring (1) plus quantities of the the acetylene (2):
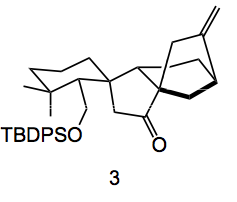
It was this observation of a side product that lead to the successful completion of the synthesis of the target. This once again demonstrates the value of identifying side products, they really give hints as to what is going on in a reaction, and in this case can also lead to a successful conclusion. Elimination of the triflate from the enol triflate provides the acetylene in 87% yield. Radical generation with AIBN, tri-n-Butyltin hydride provides the desired bicyclo[3.2.1]octane, 3, in 70% yield.
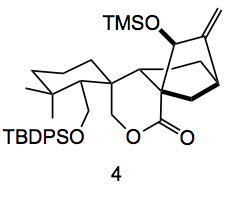
Still, after this windfall the conversion to the desired product was still very challenging. Oxidation of the ketone enolate with MoO5.Py.HMPA produced alpha-hydroxyketones followed by oxidative cleavage produced lactones, but not the desired ones. Allylic oxidation of compound 3 with selenium dioxide followed by formation of the TMS enol of the cyclopentanone and then ozonolysis followed by lithium borohydride reduction gave the lactol 4:
This actual conversion was in reality very difficult to achieve, in contrast to what one sees in the available literature. In this case a 47% yield of 4 was obtained, indicating the difficulty of this conversion. From 4 to the final product was carried out by de-protection followed by allylic oxidation.
This is a really nice synthetic route, fortunately after the initial failure of the Diels-Alder reaction a convenient bypass was utilised to obtain the desired system. Also astute observation of side products was important for the obtention of the final product. The authors must be congratulated on their tenacity as a total synthesis of this magnitude is not easy task. There is lots of interesting chemistry to take home here and I’m sure we will see more like this in the future.
4,023 total views, no views today