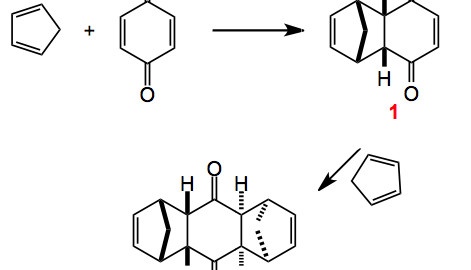
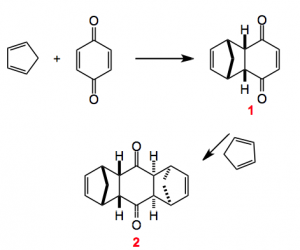
This weeks choice comes from the pages of Angewandte Chemie and is a review by Christopher Nawrat and Christpoher Moody highlighting the use of quinones as dienophiles in the Diels-Alder reaction.
The review also provides us with some of the history behind this reaction which turns out to have been discovered some twenty years earlier but its potential was not recognised. Interestingly Diels and Alder in their publication warned the competition off by “explicitly reserving the entire area of this chemistry for themselves”. Now, I wonder what would happen today if this were to appear in a publication somewhere. I bet it would invoke a storm of protest but back then it seemed to work and as we know these two chemistry received the Nobel prize for their discovery. Once they were awarded with the prize the floodgates opened and the chemistry community got busy. Notably Stork and also Woodward (who else?) published their syntheses of cantharidin and desoxycantharidine respectively.
Woodward employed this reaction many times in his formal total syntheses of various steroids and alkaloids notably reserpine. His interest unlimitedly resulted in the formulation of the Woodward-Hoffmann rules of orbital symmetry allowing an understanding of the observed product stereochemistry.
The review documents the utility of quinones in the Diels-Alder reaction in the syntheses of many natural products over the years. For anyone interested in a bit go the history of organic synthesis this article is a worthy read.
2,458 total views, 2 views today