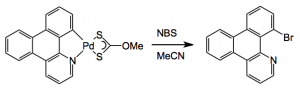
Fujita and his group have been soaking again this time using a crystalline flask to x-ray a reaction in progress. The reaction they studied was Pd catalysed aromatic bromination:
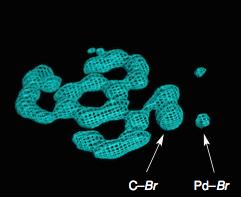
They were able to do this reaction with a single crystal embedded in a scaffold and take x-ray pictures as the reaction proceeded. Now to the ordinary organic chemist this is a great achievement, do your reaction and immediately get an x-ray of the product, saves money on expensive NMR machines! Here are a couple of the pictures they obtained
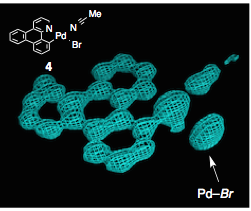
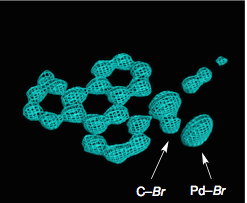
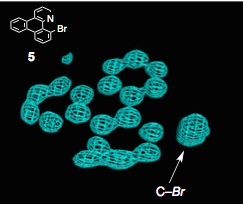
These are electron contour maps taken as the reaction happened, at the start, after 6 hours, then 15 hours and at the end. Note that the Pd ligand has changed from the xanthate to the acetonitrile case. They clearly show the presence of electron density which they attributed to the formation of a C-Br bond at the expense of a Pd-Br bond. Now I’m no expert but that looks quite convincing to me. The reaction progress was also followed by Raman spectroscopy, which confirmed this assignment. The authors comment “Accordingly, we were able to directly visualize, via X- ray snapshots, the eventual conversion of intermediate 4 to the final product 5 (Figure 4). Namely, electron density corresponding to the Pd(II)−Br bond gradually disappeared and was replaced with the emergence of density at the aryl−Br bond. Throughout the crystallographic observation of the reaction center, the square-planar geometry of the palladium center was maintained, and no apically coordinated Pd(IV) species were observed. It should be mentioned that our X-ray technique using crystalline flasks has a limitation in that fleeting species with low populations are hardly observed because of their trivial contribution of the changes in the electron density maps”.
I think that this is a wonderful result and it opens up all sorts of opportunities for mechanistic studies of solid state chemistry. If you see an interesting intermediate appearing then you just cool the system down and the reaction stops allowing you to determine the structure. The possibilities are endless. I look forward to more such publications from this group showing the applications of this methodology.
I wonder what the single crystal yield was?
The pictures were borrowed from the supplementary material, thank you.
2,479 total views, 1 views today