Nosylates (p-nitrobenzenesulphonyl) protecting groups for alcohols are well established in organic chemistry. In a recent synthesis of (-)-lepistine by Fukuyama etal they have been put to good use.
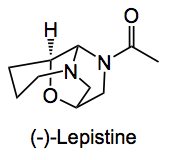
This compound was isolated from a mushroom. It has an acylated aminal and an odd arrangement of rings. According to the authors no information is available either on previous synthesis or biological activity. In this paper they present us with a synthetic approach to the ring system of lepistine.
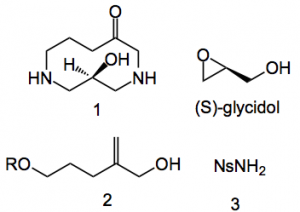
Conducting a retrosynthetic analysis they arrived at compound 1, where the amines are in a protected form, this can be accessed from (S)-glycidol, compound 2 and nosylamine 3.
The protecting group chosen for the amines in 1 were noysl. Compound 1 is easily constructed in 8 steps from (S)-gylcidol and compounds 2 & 3 in 10% overall yield. An interesting fact emerged in the last step of this sequence:
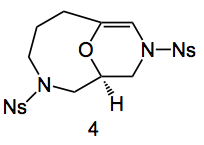
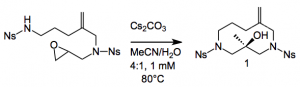 The addition of water was essential for this epoxide ring opening, no water = 8% yield with water = 70% yield. Something to bear in mind the next time you attempt a nucleophillic epoxide ring-opening reaction. Ozonoylsis of this compound afforded a hemiacetal which was dehydrated by refluxing in xylene in the presence of CSA to give the bridgehead enol ether 4 in 83% yield.
The addition of water was essential for this epoxide ring opening, no water = 8% yield with water = 70% yield. Something to bear in mind the next time you attempt a nucleophillic epoxide ring-opening reaction. Ozonoylsis of this compound afforded a hemiacetal which was dehydrated by refluxing in xylene in the presence of CSA to give the bridgehead enol ether 4 in 83% yield.
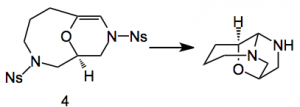
Treatment of this compound with thiophenol and caesium carbonate in acetonitrile produced the lepistine framework.
This cyclisation proceeds via a series of iminium ion intermediates generated by protonation of the enamine resulting from the PhSH/carbonate treatment of 4. Acylation of the secondary amine completed the 12 step synthetic sequence.
This synthesis demonstrates the advantages in using a single protecting group. This can’t always be the case but for a simple molecule like this one it is a good strategy and the result is an appealing short route to a quite complex system.
Edit: As pointed out by one of the more astute readers the nosylate employed here is the ortho-nitrobenzenesulphonate, not the para as I have drawn . Thank you.
4,672 total views, 1 views today
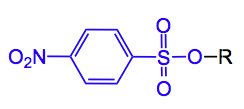
Sorry for nitpicking, I think the used nosylate is actually ortho-substituted, not para – please have a look at the supporting info.
I like this NsNHAlloc as a phthalimide equivalent, with possibility of stepwise monodeprotection/alkylation/second deprotection.
Thanks to milkshake for pointing this out. It is indeed the ortho-nitrobenzenesulphonate that has been used here.