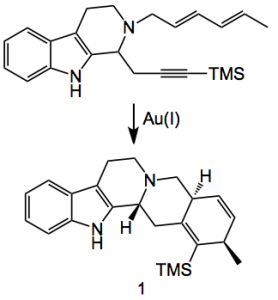
This weeks contribution has a golden touch to it and deals with the preparation of chiral cyclic alkylamino carbene-gold(I) complexes (CAAC-Au). They were discovered by collaboration between Prof. Waldmann at the Max-Plank institute and by Prof. Strohmann at the Technical University of Dortmund. They were trying to synthesise novel yohimbine analogues by the gold catalysed cyclisation of ene-ynes:
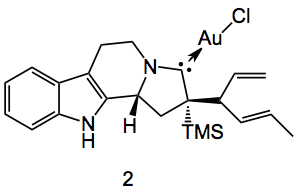
Instead of compound 1 being formed they obtained the gold N-heterocyclic carbene 2 in 90% yield:
The structure of 2 was confirmed by x-ray. In the words of the authors: “This unique gold carbene complex displays interesting structural features that include: 1) the presence of a skipped diene side chain, indicative of allylic transposition of a 2,4-hexadien-1-yl moiety and 2) the perturbation of the position of the TMS group with respect to the alkyne terminus suggests that a 1,2- silicon shift had occurred. These features clearly indicate that a multistep cascade process must have occurred involving gold-free enynes and leading to CAAC–AuI complexes (2). To the best of our knowledge, chemical transformations involving an intramolecular allylic alkylation of this type with gold salts as substrates have not been reported before.”
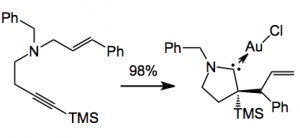
They then went on to explore the scope of this novel cascade process with a variety of functionality on the original ring system and obtained the corresponding gold carbene complexes in good yield. So the allylic alkylation seems to be quite general. It even extends to acyclic substrates:
The reaction seems to be very simple to carry out: You add a dilute solution of 1 equivalent of (Me2S)AuCl in 1,2 dichloroethane to the cyclisation precursor and warm to 40°C with the exclusion of light for 18 hours and isolate your product. The only thing is the dimethylsulfide gold(I) chloride reagent is rather expensive, 1 g costing around £120 (presumably depending upon the price of gold).
The authors propose a mechanism for this reaction:
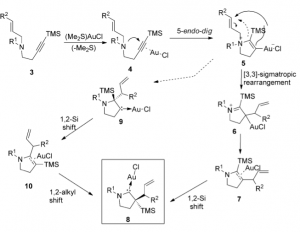 which involves a cyclisation/re-arangement sequence. These gold carbenes are catalytically active and promote the cyclisation of N-methyl-2-(2-phenylethynyl)aniline to the corresponding indole in high yield.
which involves a cyclisation/re-arangement sequence. These gold carbenes are catalytically active and promote the cyclisation of N-methyl-2-(2-phenylethynyl)aniline to the corresponding indole in high yield.
A further interesting aspect of the N-heterocyclic carbenes is their potential to inhibit cancer cell growth. The compounds described in this paper have additional biologically active structural features (alkaloid scaffolds) which are often active against cancer cells. Indeed the human ovarian cancer cell line A2789 proved to be very sensitive towards the gold carbenes in sub-micromolar concentration.
So apart from discovering a novel cyclisation-rearrangement sequence and novel chiral catalysts the authors have opened up a possible avenue for med. chem. programs to optimise the promising biological activity. Congratulations on a very nice piece of work.
4,633 total views, 1 views today