It’s nice to see that people still have an interest in synthesising discodermolide. This week’s ASAP describes a new way of preparing the (Z)-tri-substituted double bond at C13-C14, which was a killer step in our synthesis giving only 30% yield. However the Morken group may have found a way around this low yielding step. by employing catalytic stereoselective borylation chemistry.
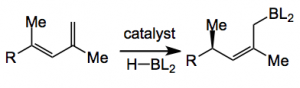
Their idea was to employ a Ni catalysed 1,4-diene hydroboration to establish the (Z)-tri-substituted double bond:
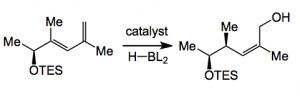
Morken had already demonstrated the success of Ni catalysed 1,4 -hydroboration of such olefins with a Z/E ratio of >20:1. Unknown was the effect of neighbouring chiral centers on the diastereoselectivity of the reduction. So a number of 1,4-dienes were prepared and hydroborated. With various chiral R groups one interesting result was the following:
The catalyst was [Ni(cod)2] 2.5%, PCy3, 2.5% using HB(pin) in THF. The reactions were run at 0.25M concentration of diene (somewhat dilute) and after work up gave a 93% yield of the allyl alcohol in >20:2 Z/E with a d.r. of 12:1. So an interesting result. Further extension to discodermolide requires the oxidation of the primary alcohol and some sort of stereoselective enolate coupling to form the C15-C16 bond. Remembering that quite a few synthesis have stumbled upon this very reaction as crucial and obtaining excellent diastereo-control is not easy. So iodide 1 was prepared in 65% overall yield in 4 steps from (allyloxy)(tert-butyl)dimethylsilane which involved a hydroformylation reaction in the presence of (S, S)-PhBPE, chelation controlled addition of a vinylzinc compound and finally the hydroboration reaction, TBDMS protection of the alcohol and iodination of the primary alcohol:
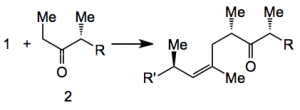
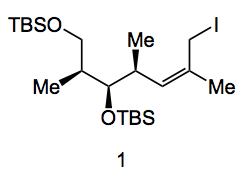 Now comes the crucial coupling reaction (I’ll only show the reactive parts of the molecule):
Now comes the crucial coupling reaction (I’ll only show the reactive parts of the molecule):
Using conditions developed in 1991 by Collum etal. the E enolate of the ketone 2 was selectively prepared by treatment with lithium tetramethylpiperidide in the presence of lithium bromide at -78°C. Addition of 1 equivalent of the allyl iodide produced the product in very high yield and stereoselectivity (82% and 10:1 d.r.). A further 8 steps eventually gave discodermolide. Total 36 steps, 17 in the longest chain, in 13% yield.
Now this approach should allow easier synthesis of analogues, especially of the C13-C14 C=C. This double bond is the cause of the hairpin shape of discodermolide and contributes to it’s biological activity. Until now it has been difficult to insert other substituents on this double bond, other than methyl or H, especially with the Zhao olefination we used.
This synthesis should be amenable to scale up in spite of using significant quantities of various transition metal catalysts and a step involving carbon monoxide. The use of reactive organo zinc reagents may well also prove problematic. Unfortunately the supplementary material for this paper seems to be incomplete, so an exact analysis is not possible. However this is an interesting route to discodermolide. Well done on a novel construction method.
3,108 total views, 2 views today
Oh yes, the SI is rather lacking…
Presumably Ni(0) means glovebox for the hydroboration?
Yes it’s glove box chemistry.
I’ve managed to obtain a copy of the complete SI from the authors. I shall comment on it soon.