This week’s pick involves a reagent that one does not often see used in organic synthesis, which is laughable really, considering it’s potential: nitrous oxide. It was reported here by a group from the ETH Lausanne led by Prof. Severin. The chemistry leads to the synthesis of triazenes which may have a use as cytotoxic agents. A comparison of the new route and the older methods is:
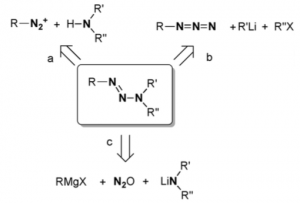
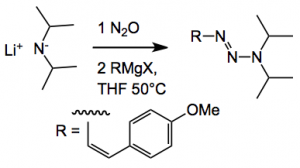
So we had diazonium salt chemistry and azide N alkylation, both very tricky to do. The new method relies on nitrous oxide “mediating” the coupling of Grignard reagents with lithium amides. This allows the synthesis of triazenes with valuable, in a biological sense, set of functionalisation stuck on the nitrogen atom. An example:
The triazene was obtained in 57% yield and characterised by x-ray. So what’s going on here? The authors suggest *The crystallographic analyses along with the NMR spectroscopic and elemental analyses data provide strong evidence that covalent N2O adducts with the formula R2N(N2O)Li are formed. So far, well characterised covalent N2O adducts of organic compounds with intact NNO groups have only been reported for N-heterocyclic carbenes and frustrated Lewis pairs. The ability of dialkylamides to form N2O adducts in a clean and quantitative fashion is essential for the success of the overall coupling reaction. The subsequent reaction of the R2N(N2O)Li adducts with Grignard reagents requires cleavage of the N-O bond, which is likely facilitated by the oxophilicity of the Mg2+ ions.”
The yield of this particular triazene is 57%. Generally with substituted acetylenes the yields are higher >80%. Using simple alkyl Grignards the yields dropped to around 10% due to significant side product formation (not defined). The reactions were carried out on a 2 mmol scale and the yields reported are isolated yields. In the supplementary material there is an observation that during the attempted isolation of one of the R2N(N2O)Li intermediates explosive decomposition was noted! This may limit the use of this methodology on a larger scale.
So an interesting synthetic method which will no doubt find its place. Anyway I bet they all had a good laugh while doing this chemistry.
2,894 total views, 1 views today