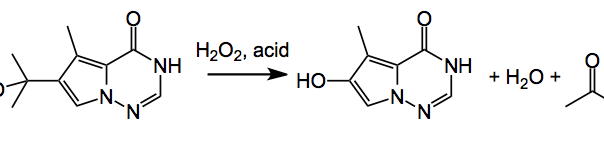
This week’s ASAP presents us with some process safety issues identified by LaPorte etal from BMS. It appeared here and discusses the development of a continuous flow process designed to avoid a thermal runaway. The reaction under investigation is:
It is a peroxide mediated acid catalysed benzyllic rearrangement which proceeds via a hydroperoxide intermediate. As an example of the potential of this reaction; a batch reaction with a 20 wt% solution of the tertiary alcohol with 12 equiv. hydrogen peroxide and a catalytic amount of HCl had an onset temperature of decomposition of 25°C which gave rise to a pressure increase of >5000 psi/min with a self-heating rate of 3000°C/min. These are quite impressive figures. There are lots of reaction components and or intermediates here which contribute to this extreme reactivity. hydrogen peroxide itself, the hydroperoxide intermediate(s), the product, acetone and so on. So a thermal runaway reaction might not use or generate hazardous chemicals but may, under certain conditions, generate large amounts of gas and heat. The rate of heat produced then exceeds the rate of heat removal at or near some critical onset temperature (decomposition). These are highly energetic reactions, and the energy released may be due to the desired reaction, degradation of reactants or products, side reactions, or autocatalytic reactions. So not for the faint hearted and even on a small scale quite exciting.
So how do you avoid this scenario? Well you begin by examining all the relevant reaction parameters you can think of and abandon the idea of using a batch production process to produce this material. Think flow! But this still needs lots of optimisation. For example the acid catalyst. Methanesulfonic acid was much better than HCl, it produced less product degradation and the self heating rate was also lower.
If you are going for a flow process then homogenous solutions are a must and any precipitation of products or intermediates should be avoided, for obvious reasons. You need to have rapid reactions and the reaction should be done at a temperature at least 10°C less than the onset temperature. Quenching is also an important part of the sequence and it was found that the product is not stable to the reaction conditions. So after completion the RM was added to a large volume of the reductive quench (sodium bisulfite) maintaining the pH at 7.2 using aqueous ammonia.
However after more intensive study the authors designed a flow reactor for their kilo-lab. This was able to produce around 6 kg of product per day! Further scaling eventually ended up with a system suitable for manufacturing. Here is a summary.
Really very impressive figures here given the nature of this reaction. Thus “A two-stage temperature control strategy was employed to maximize safety and efficiency while producing high quality and high yielding product. Steady-state operation was achieved quickly and maintained throughout processing. More than two metric tons of the hydroxypyrrolotriazine product have been produced at commercial scale utilizing this continuous process. The process was demonstrated for up to 33 days of continuous operation with no safety- or quality-related incidents.“
This is a great piece of work which has been presented, and shows just some of the problems one can encounter in process development. The elegant solutions presented should be an inspiration for others facing similar problematic steps in their synthetic routes. Congratulations.
3,154 total views, 1 views today

I would try to immobilize the acid catalyst (Amberlyst or Dowex RSO3H) and pump the peroxide+starting material mix through it. By the way, this reaction has a clear precedent in fragmentation of cumene hydroperoxide (Dow process for making phenol and acetone). I am sure a process making commodity chemicals must be exceedingly well optimized, maybe they could just use that setup
Thanks for the comment. Yes, I’m not sure I’d have done it this way either. Their intermediate is obviously not such a great one to work with so maybe they tried the Dow process and ran into trouble?
Anyway it may be that they are the flow group in BMS and have to justify their existence!