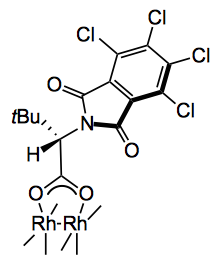
This week a paper from Waldmann appeared discussing the enantioselective rhodium catalysed cycloaddition of tropone and carbonyl ylides:
Through a catalyst screening sequence the best catalyst was :-
At the 1 mol% level it produced a 74% yield of the product in 99 %ee. The catalyst load can be reduced without affecting the %ee but the reaction times are longer. Note that the solvent here was trifluoromethylbenzene.
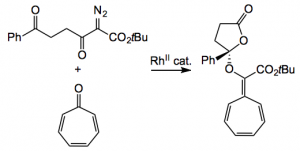
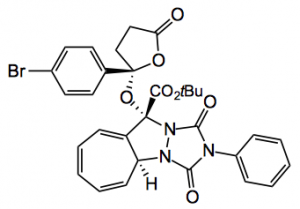
A range of diazo-diketo derivatives was examined by varying the nature of the phenyl group. The yields were all around 70% with extremely high %ee’s. The mechanism of this reaction was proposed to proceed by a [3+2] cycloaddition of a carbonyl ylide and the carbonyl of the tropone. The product was also further functionalised by an [8+2] cycloaddition to give the following product:
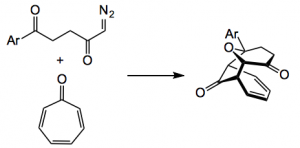
Diazo ketones give a different product, one of a [6+3] cycloaddition:-
The cycloadduct was produced in around 90% yield with a variety of Ar groups. The same catalyst was used, 2 mol% in trifluoromethylbenzene. The optical purity was high mostly over 90 %ee. This presumably arises because of the “…… reactivity difference arising from the different HOMO/LUMO energy levels of carbonyl ylides derived from diazodiketoesters and a-diazoketones.
So interesting simple chemistry from Waldmann exploiting the differing reactivity of tropone depending upon the substrate employed. I’m sure this will re-appear in the future given his interest in biology orientated synthesis. Somewhere there must be a natural product whose synthesis just cries out for this chemistry.
1,841 total views, no views today