This week another new use for older compounds from the Baran labs. The ASAP discusses the use of quinone diazides in steroid synthesis.
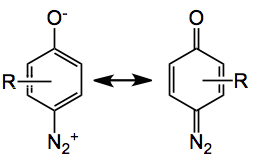
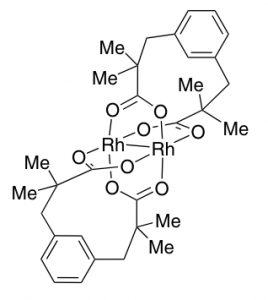
Now these things have been around for quite some time and are simply made by diazotisation of the corresponding aminophenol. So here we have a carbene source, what is the best way to generate this particular one? Certainly rhodium catalysis is asked for and several were examined in a model reaction with cyclooctene. The best was [Rh2(esp)2] at a level of 5 mol% in dichloromethane producing the cyclopropane in reasonable yield, 66%. The catalyst structure is:
and is readily available.
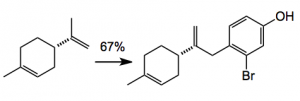
With a cyclopropane in hand the next step was to investigate the selective C-C bond cleavage process, as several products could result. The authors managed to find conditions which allowed this selective cyclopropane opening by bringing the “quinone part of the molecule into play”, for example:
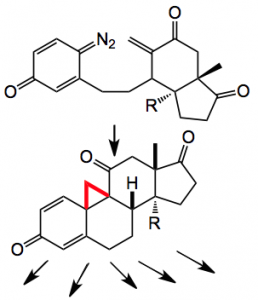
So far so good. In order to really make use of these carbene precursors a convenient precursor was required, one which allows the generation of the diazide under mild high yielding conditions, not at least when one is considering their use in the synthesis of steroid frameworks:
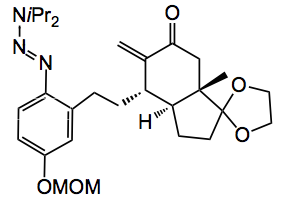
From the authors” Since its resonance structure is an oxyaryldiazonium zwitterion (above), it was anticipated that methoxymethyl (MOM) and triazene groups would effectively shield the oxyanion and diazo species, respectively, and be easily unmasked under mildly acidic conditions. This masked quinone diazide was anticipated to arise from the union of triazenes with trans-bicyclic enones by conjugate addition followed by trapping with a one-carbon electrophile.” Through a convergent route the following triazene was synthesised:
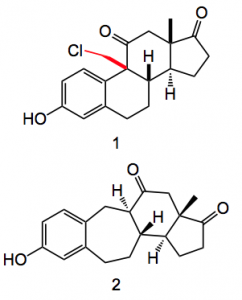
Treatment with TFA unmasked the diazide which was treated with the rhodium catalyst to give a high yield of the cyclopropane (drawn above). Reaction with lithium chloride produced the expected aromatic steroid system, compound 1 and hydrogenation gave the ring expanded system 2. Whose structures were confirmed by x-ray.
From the authors”In conclusion, this work represents the first systematic study of quinone diazides, a ubiquitous entity in materials science, in the context of organic synthesis. The most significant findings resulting from this inquiry include: 1) a general rhodium-catalyzed intermolecular cyclopropanation of quinone diazides with a variety of different olefin classes, 2) divergent manipulations of the resulting cyclohexadienone adducts, 3) a strategy to enlist quinone diazides in complex intramolecular settings using a triazene-enabled “mask”, and 4) application to a fundamentally different approach to the steroid skeleton.”
So there we have it another wonderful bit of chemistry from the Baran labs. Now I’m not an expert in steroid synthesis, but I guess that this approach may have had Fieser jumping in his chair. The approach that Baran and his group have to their chemistry I find very refreshing and it is always a joy to read the ensuing publications.
4,923 total views, no views today