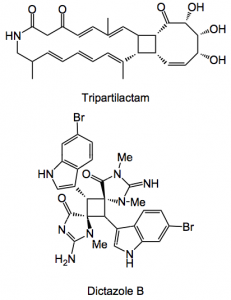
In a JOC article Baran and Gutekunst discuss the use of C-H functionalisation in the synthesis of substituted cyclobutane rings. Why cyclobutanes? Well there are lots of natural products containing this ring system, two examples:
Amazing what nature can produce. So with an increase of interest in the biological activity of such molecules a scarcity of synthetic methods has med itself apparent. Just look at the [2+2] photocycloaddition, this alone can produce 11 possible isomers. Now obviously not all are formed depending upon the olefins and the conditions, but still! The NMR is also a nightmare where it is apparently easy to mis-assign peaks due to rapid ring flip. This review type article is chock full of fascinating examples of synthetic chemistry and is definitely worth deep study.
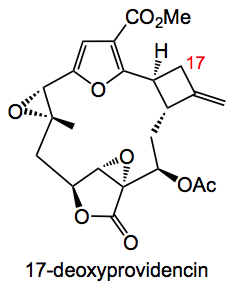
A contribution from the Mulzer group in Vienna also deals with the manipulation of cyclobutane rings in a presentation of the synthesis of 17–deoxyprovidencin:
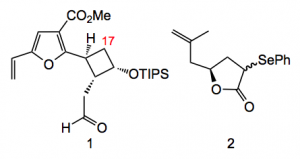
Placing a protected alcohol at the site of the exocyclic C=C and disconnecting the molecule via a RCM and an aldol gives the two fragments 1 & 2:
Compound 1 can be made in 11 steps and about 20% yield from cis-bicyclo[3.2.0]hept-2-en-6-one which is readily available, so the cyclobutane ring comes already supplied. The other part stems from glycidyl tosylate. Construction of the macrocyclic framework proceeded uneventfully, as they say, and time had come to attempt the first epoxidation reactions.
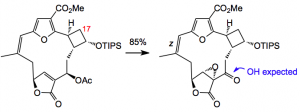
Reaction of the vinyl acetate with basic hydrogen peroxide produced an epoxide but the acetate had been converted into a keto group. I suppose this is one of those moments that synthetic organic chemists have. Not to be outdone a mechanism for this transformation was suggested involving a 1,4-addition of a hydroperoxide anion which through a series of steps collapses to produce the epoxy-ketone observed. With that anomaly explained a different set of conditions for epoxidation was examined: NaOCl/water/pyridine at -15°C this produced the desired epoxide in 87% yield:
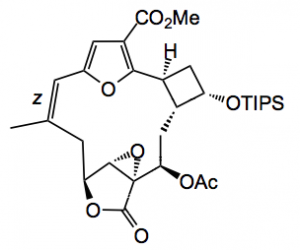 This was confirmed by x-ray diffraction. Photoisomerisation of the Z C=C to the E isomer demonstrated that the wavelength of the light is crucial as the absorption maximum of the epoxy acetate is at 306 nm and the isomerised E olefin lies below 300 nm so pyrex glass was used (cuts off wavelengths below 300 nm) instead of the usual quartz glass. H-NMR analysis proved difficult due to the formation of atropisomers at RT. Heating to 75°C caused this to slow and produce sharp signals so that the E configuration of the olefin could be confirmed.
This was confirmed by x-ray diffraction. Photoisomerisation of the Z C=C to the E isomer demonstrated that the wavelength of the light is crucial as the absorption maximum of the epoxy acetate is at 306 nm and the isomerised E olefin lies below 300 nm so pyrex glass was used (cuts off wavelengths below 300 nm) instead of the usual quartz glass. H-NMR analysis proved difficult due to the formation of atropisomers at RT. Heating to 75°C caused this to slow and produce sharp signals so that the E configuration of the olefin could be confirmed.
Once again another great example of total synthesis from Johann’s group. If you ever get a chance to visit Vienna, go pay them a visit. I remember Johann’s office as being in one of these very old Viennese imperial buildings where the roof of the room seemed to vanish into outer space.
5,819 total views, no views today