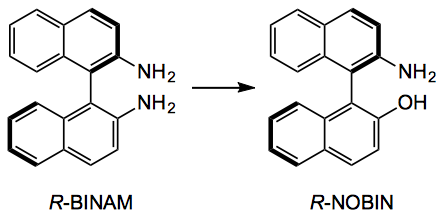
Atropisomers such as biaryls are not without their uses in organic synthesis and I’m sure we are all aware of them. Normally they have their coordinating functional groups intact and if you need a different set, well you may have some trouble. This weeks ASAP presents a method for the functional group conversion of biaryls circumventing some of the tedious synthetic methods normally employed.
Armstrong and his colleagues from the University of Texas at Arlington, Arlington, United States. They studied this reaction-
So R-NOBIN is very expensive (about $1000/g) and R-BINAM cheaper ($140/g). So for cash strapped departments this could be an interesting conversion. So heating the diamine in 1.7M HCl in dioxane/water 2/1, 85°C for 6 hours and adding 2.7 equivalents of benzoyl peroxide gave a 70% yield of NOBIN. No racemisation observed.
The report details the extensive optimisation experiments. The authors suggest that this is an example of a radical Bucherer reaction. “Radical-Bucherer reactions have not been described in detail; however, it is possible that a radical pathway leads to either the imine tautomer or oxidised (semiquinone-like) radical imine of BINAM. These species should undergo hydrolysis under equilibrium and rearomatisation to the 2-naphthol group by a mechanism related to the Bucherer reaction.” I haven’t come across such a reaction since university times.
So this has the potential to generate even more chiral biaryls as metal coordinators for asymmetric catalysis.
2,601 total views, 1 views today