The direct functionalization of C-H, that is, the formation of C-C or C-Heteroatom bonds has always been an important tool in the box of organic chemistry methods. Between 1991 and 2011, the number of papers appearing per annum has gone from <100 in 1991 to >500 in 2011. The applications of this methodology to the synthesis of natural products and pharmaceuticals would allow rapid access to these biologically active classes of compounds
Until recently is has, with the exception of aromatic chemistry, been restricted to a few reactions, for example, Grignard chemistry, proceeding via a halo-organic species, the reaction being functional group intolerant. Or the selenium dioxide induced allylic oxidation and ortho-metallation, which uses very strong bases, both methods employing stoichiometric quantities of reagents. Of greater interest is the use of catalytic procedures to achieve this functional transformation.
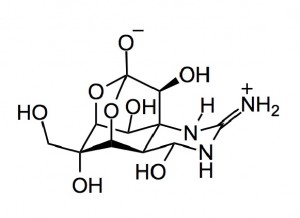
A striking example of the utility of C-H functionalisation (CHF) is demonstrated by the synthesis of (-) tetrodotoxin. Isobe etal published a 67-step route in 2003. The step count was reduced to 32 by Hinman and DuBois employing a C-H insertion and amination approach.
(-)-Tetrodotoxin:
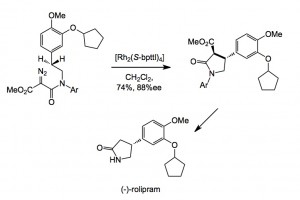
 A useful method for CHF is the rhodium-catalysed insertion of a carbene into a C-H bond, the carbenoid precursor being a diazo compound. However, with the advent of chiral rhodium catalysts, the chiral version of this reaction began to be investigated. The following scheme is a nice example in a synthesis of (-)-rolipram:
A useful method for CHF is the rhodium-catalysed insertion of a carbene into a C-H bond, the carbenoid precursor being a diazo compound. However, with the advent of chiral rhodium catalysts, the chiral version of this reaction began to be investigated. The following scheme is a nice example in a synthesis of (-)-rolipram:
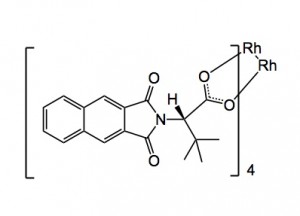
The catalyst has the following structure:
This well documented carbene generation with rhodium complexes becomes even more synthetically relevant by the publication of a recent paper by Prof. Raines, at the University of Wisconsin, describing the formation of diazo compounds from azides in water, thus making much more structurally complex diazo compounds synthetically available.
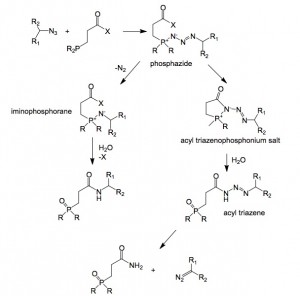
Apart from carbene formation diazo compounds can undergo a variety of other synthetically useful reactions, for example, they undergo 1,3-dipolar cycloadditions with a wide range of dipolarophiles. Protonation of the carbon atom produces diazonium ions, which are excellent alkylating agents. Harsh conditions are normally required for their formation; this can lead to decomposition and undesired side reactions. The mechanistic rationale for the conversion of azides to diazo compounds in the presence of a phosphine is outlined in the next scheme (taken from the publication.
Diazo formation:
From the phosphazide there are two possible decomposition pathways depending upon the capacity of the acyl group to trap the phosphazide before loss of nitrogen occurs. Raines surmised that this ability was dependant upon the pKa of the conjugate acid of the leaving group. Thus more reactive acyl groups favour diazo formation and less reactive the pathway leading to the amide.
This hypothesis was shown to be correct in that the diazo/amide product ratio correlated with the pKa of the conjugate acid. If the pKa was >9.2 then nitrogen elimination was favoured, lowering the pKa to <7.6 produced exclusively the diazo compound.
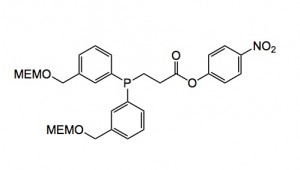
The most efficient water-soluble phosphine was the compound shown below:
Treatment of azides with this compound in a 50% v/v methanol/10mM sodium phosphate buffer at pH 7.0 produced an 80% isolated yield of the diazo compound after chromatography on silica gel.
Broad ranging investigations ensured the compatibility of the phosphine with a large variety of functional groups as well as various biomolecules, for example, oxidised L-glutathione, a molecule that is present in many cells and contains nucleophilic and electrophilic groups.
One could imagine many applications for this methodology in biology and chemistry, which I will not divulge here but leave the reader to use his/her imagination. The only criticism that I have of this work is that examination of the experimental procedures reveals that the reactions were carried out on a very small scale, 0.070 mmol. It would have been nice to read about some of these reactions on scale, 1-25 or more grams. I don’t see a problem with the diazo group formation in such a case, however the synthesis of the phosphine, as described on scale may cause a few problems.
All in all a very nice piece of work. Congratulations.
4,340 total views, 1 views today