I think everyone has heard of the Hajos–Parrish–Eder– Sauer–Wiechert reaction which was discovered In 1971 and demonstrated the efficacy of L-proline as catalyst for the intramolecular asymmetric aldol cyclodehydration. Starting with an achiral triketone and just 3% of L-proline, the product, a ketol, was produced in 93% enantiomeric excess. As usual this result was ignored for many years and re-discovered many (almost 30) years later. It was immediately defined as the acceleration of a chemical transformation through addition of a substoichiometric amount of an organic compound which does not contain a metal atom.”
A recent review written by Abbasov and Romo of Texas A&M University highlights developments in this area from 2010 to 2014. They place an emphasis on scalable versions of the organocatalytic methodology with reference to the synthesis of natural products. Now I’ll bet that my definition of scalable and theirs differs somewhat, but never mind.
There has been a classification of the asymmetric modes of activation of the organocatalyst into covalent and non-covalent depending upon the type of interaction with the substrate. So we have things like iminium catalysts, enamine forming catalysts, acylammonium, N-heterocycloc carbenes and so on. Non-covalents are things like phase transfer catalysts, chiral anion (phosphate) catalysts, Brønsted acids and so-on. So recent examples of asymmetric iminium/enamine catalysts are –
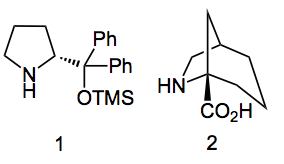
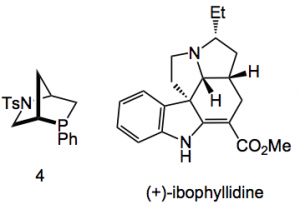
Compound 2 was used to asymmetrically alkylate 2-phenylpropionaldehyde with benzyl bromide and gave an 80% yield of compound 3 with an ee of 91%.
Now this is ok, until you realise that 2 was used in 30mol% quantities. Is that really catalytic? Although it fulfils the definition 30mol% is not catalytic in the true sense of the word catalytic. Now a quick look in PubChem gives 4 vendors for this “catalyst” and I found another who sells it for $2,250/g. At that price you’d better to be able to recover it for the next catalytic reaction. Most of these types of catalysts are used within the range of 10 – 30mol%. They are also very useful in catalysing the Diels-Alder reaction.
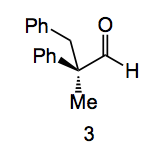
Chiral phosphine ligands have always played an essential role in catalysis invariably attached to a transition metal. They can be used for catalysis in their own right, for example phosphine 4 catalysed a [3+2] annulation as part of a synthetic sequence which provided (+)-ibophyllidine:
Phosphine 4 was used at the 10mol% level and gave the intermediate in 94% yield and 97%ee. This is a very good result, but I think it is one of the better ones, the ee% seems to be a bit lower in most cases.
As is usually the case the results reported in this review stem from academic labs where each intermediate was purified by column chromatography. Nowhere to be seen is a comment about catalyst re-cycling, which, at the prices quoted for the above example, may be vital. If you have prepared the optimal catalyst yourself re-cycling the 30mol% will become even more important. I feel that these details are missing from such papers and their supplementary info. Correct catalyst selection is also vital and an efficient screening is required to find the one for your reaction, but this is the case for most catalysts.
So organocatalysis is here to stay and provides yet another tool for the synthetic organic chemist to play around with. Do use it if you can and mention the re-cycling in your resulting paper.
2,241 total views, 1 views today