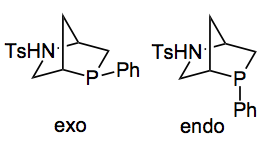
Continuing on the theme of organocatalysis this week’s ASAP appeared in JACS and is the result of a collaboration between Kwon from the University of California, Los Angeles and Dudding at Brock University, St. Catharines, Ontario. It presents a nice solution to a problem that was also dear to my heart a few years ago, the asymmetric synthesis of pyrrolines. At that time I was interested in achieving an enantionselective approach to a very similar class of pyrroline, but I never got round to thinking about the following two phosphines as catalysts:
They are simply made from trans-4-hydroxy-(L)-proline in 6 steps in 35% overall yield. They are relatively air stable, the word actually used was “sufficiently” and this was not defined in the paper and is an important missing piece of information. Already some 12 analogues have been prepared and are they now being commercialised by Aldrich.
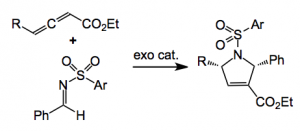
So what were they used for? The [3+2] allene-imine annulation:
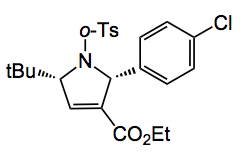
Using 10mol% of the catalyst in benzene at rt for 1 to 2 days provides the pyrroline in high yield around 90% and depending upon the Ar and R groups in high ee, 97% in the case where R = tBu and Ar = p-nitrophenyl. The enantioselectivity is enhanced by using electron deficient groups on the Ar substituent, so the nosyl was the best (97% yield, 97%ee). Conveniently this group is easy to remove. Of course employing the endo phosphine produced the opposite enantiomer so the two diastereoisomeric phosphines function as pseudoenantiomers. A good demonstration of this catalysts effectiveness is in the preparation of a chiral precursor, to a GGTase-I inhibitor, whose structure is depicted below, in 92% yield with 90ee%:
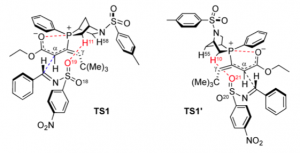
Calculations allowed the authors to identify two possible transition states for this reaction:
One can see that the exophosphine blocks the Si face of the phosphonium dienolate, while the endophosphine blocks the Re face, resulting in opposite absolute configurations at the carbon-imine atoms.
So, this represents a new class of rigid chiral [2.2.1] bicyclophosphines that are highly effective catalysts for the asymmetric syntheses of 1,2,3,5-substituted pyrrolines.It will be interesting to see what else they can catalyse in an asymmetric sort of way. Nice piece of work and a good read. I wish I’d had this one when I was dealing with this sort of chemistry. It would have made life much easier, I’m sure.
2,495 total views, 1 views today