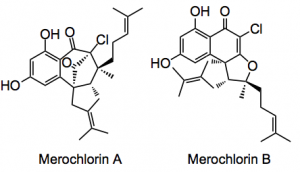
This week’s ASAP is a one pot synthesis of two complex halogenated meroterpenoid natural products with significant antibacterial activities; Merochlorins A & B.
The synthesis was conceived by a group led by Bradley Moore from the Center for Marine Biotechnology and Biomedicine Scripps Institution of Oceanography University of California San Diego with the collaboration of Baran at the Department of Chemistry, The Scripps Research Institute. The report appeared in Angewandte Chemie International edition this week. These two compounds are produced by the marine bacterium Streptomyces sp. strain CNH-189.
Four enzymes were identified that carry out this complex chemistry, a type III polyketide synthase, a prenyl diphosphate synthase, an aromatic prenyltransferase and chlorination by a vanadium dependant haloperoxidase. These two compounds have been synthesised chemically however their biosynthesis was unknown, until now.
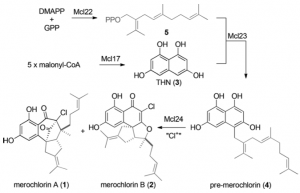
Genetic analysis of the Streptomyces sp. strain CNH-189 showed that it encodes the merochlorin biosynthetic machinery within a 57.6 kb-sized gene cluster comprising a total of 41 predicted proteins. A biosynthetic pathway was proposed:
So feeding the enzyme with dimethylallyl diphosphate (DMAPP) and geranyl diphosphate (GPP) produces compound 5 in the scheme. treating malonyl-CoA with the enzyme merochlorin 17 delivers the aromatic system which is then coupled with compound 5 to give pre-merochlorin. The vanadium dependant haloperoxidase finally produced the two antibiotics.
Catalytic mechanisms were suggested for the production of compound 5, the aromatic bit and the coupled product. By fiddling around with the genes and doing deuterium labelling experiments the authors were, after a lot of work, able to confirm their mechanistic proposal. As I don’t really quite understand the methods used I won’t comment further.
In the words of the authors:”In summary, we unraveled the biosynthesis of the structurally complex merochlorin A and B, thus allowing for the first time, the total enzymatic synthesis of meroterpenoid natural products. Astoundingly, unlike many other reconstituted secondary metabolic pathways, merochlorin A and B biosynthesis merely involves four dedicated enzymes and the common metabolites DMAPP, GPP, and malonyl CoA. This pathway represents a new example of how structural diversity and complexity are generated in nature with a limited set of catalysts.”
I think this beats the xxx step synthesis of some natural product using 99 different catalysts. But the quantities produced are very small, not enough for NMRs. I’m sure that implantation of the gene sequence into some poor E.coli system would enable the production of large quantities. Not to mention that this publication represents a tremendous piece of work, congratulations.
3,932 total views, 1 views today