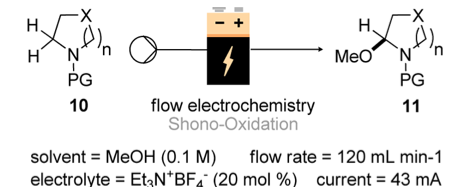
Yet another flow system from the Ley labs, this time utilising electrochemistry to prepare a few indole alkaloids. Starting off by examining the Shono oxidation of cyclic amines, N-protected.
Applicable to (n) being 0-2 and PG being Boc, Cbz, Ac and Teoc. Yields all in the lower 90% range. Using a carbon anode and a steel cathode with tetramethylammonium tetrafluoroborate as the electrolyte the system was stable and able to perform consistently over a period of 14 hours. No variation of conversion of purity was observed. So a rapid and efficient way into such cyclic amines which have excellent synthetic potential in their aminal form..
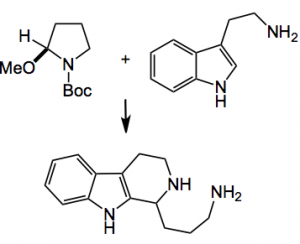
A demonstration of their use was shown by conducting a Pictet-Spengler reaction:
The optimal conditions were CSA (6 equiv.), for 30 minutes at 130°C in water. This produced an 87% isolated yield of the cyclised product. So we have a two step process delivering alkaloids in high yield.
So with this latest addition to the flow chemistry I think the majority of organic synthesis methodology is now covered by this sort of process. The contributions from the Ley group have been instrumental (pun intended) in developing this technology. So the life of the synthetic chemist has now become much less stressful; set up your pipes and pumps, starting materials in, products out, no leaks = lots of compound. Great. Wish I could get to play with this.
2,590 total views, 3 views today