The synthesis of olefins belongs to the fundamental group of organic chemistry methods. Lots of ways are available to achieve olefin synthesis, the Wittig reaction and the phosphonate equivalent, the Horner-Emmons reaction, are amongst the most utilised in synthesis. Now these methods are not catalytic, they utilise strongly basic conditions, which may cause problems elsewhere in your molecule. They produce side products, triphenylphosphine oxide for example, that are difficult to remove. Also controlling the resultant olefin geometry (cis or trans) can be a thorny problem. So it was nice to see a catalytic method recently described by the Milstein group at the Weizmann Institute in Israel.
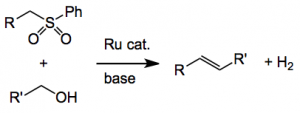
This group describe the direct one step catalytic method for the olefination of alcohols using sulfones and a ruthenium pincer complex:
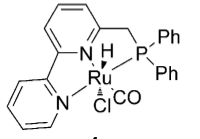
The ruthenium complex has an interesting structure:
Using this catalyst (0.025mmol), 4-methoxybenzyl alcohol (1 mmol) in dioxane at 125°C for 5 hours in the presence of dimethylsulfone and potassium tert-butoxide gave 4-methoxystyrene in 73% yield. A somewhat limited scope of the reaction, with respect to the alcohol substituent, was investigated. Only para substituted aromatics were employed and they were limited to phenyl, methoxy, methyl and halogen (Cl and F) and H (phenyl). The yields were reasonable ranging from 75 – 85% the best being R = Ph. Naphthyl both the 1 & 2 isomers also worked, the 1-naphthyl giving only 50% yield of the olefin, decanol produced a complicated mixture resulted probably due to C=C isomerisation. The effect of the R’ substituent on the sulfone was examined. Again somewhat limited in scope, the compounds used were as above but generally gave lower yields. The E alkenes were formed, but the authors did not mention if any Z isomer was formed except for the case where R’ is 6-methylpyridyl, here a 3:1 mixture of E:Z was formed.
One of the side products from this reaction is hydrogen. No olefin hydrogenation was observed because the reaction system was open. Carrying it out in a closed system resulted in hydrogenation, and is therefore a method for the direct conversion of RCH2OH to RCH3. Deuterium labeled dimethylsulfone confirmed that the olefinic methylene group in the styrenes indeed originates from the sulfone. Now one may expect the intermediate formation of an aldehyde, however, when benzaldehyde was used only a 10% yield of the styrene was obtained. Other results also suggest that the aldehyde is not “really” present but may be coordinated to the metal.
So this may be a useful method here. I would have liked to have seen a larger range of alcohols studied because at the moment it seems limited to substituted benzyl alcohols. I wonder what happens with chiral benzylic alcohols that would be interesting to find out. I also get the impression that the minimum was done here to qualify for a publication and in the rush they missed out on the reaction scope.
Here we have another synthetic tool which will find its niche.
2,871 total views, 3 views today