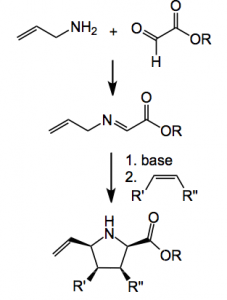
This week’s offering deals with a reaction which I experimented with several years ago (more later), the well studied [3+2] cycloaddition of azomethine ylides with alkenes producing pyrrolidines. Normally the ylide is derived from the imine of an amino acid however Waters and his group now report a novel entry to produce azomethine ylides starting from allylic amines and glyoxals.
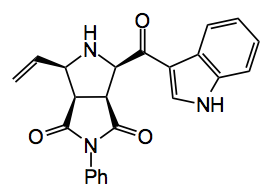
Normally these imines are not acidic enough to form the ylide, however, in the presence of a metallic catalyst triethylamine is sufficient to form the ylide. Silver acetate is the catalyst of choice here and Waters used 10 mol%. By carefully optimising the reaction conditions he was able to obtain the pyrrolidine after cycloaddition with the ylide derived from benzylamine and phenyl maleimide as a single diastereoisomer in good (86%) yield. One example of the kinds of pyrrolidine he was able to produce is given below.
So quite a useful method and it removes the reliance on amino acids for the success of this chemistry.
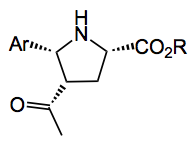
My interest in this chemistry was in obtaining the following compound, for reasons I won’t go into here:
But I was stuck with the amino acid ester approach, but was able to prepare a large series of imines from ArCHO, most of which were stable and if you chose the correct ester, i.e. t-butyl the imines were crystalline. So using 1.0 – 1.5 equivalents of AgOAc I obtained a 40 – 50% yield of the pyrrolidine after reaction of the imine with methyl vinyl ketone. As was also noticed by Waters a side product is formed namely the N-alkylated compound formed by conjugate addition of the product with methyl vinyl ketone. In my case I was able to crystallise product and the N-alkyl compound remained in the mother liquors.
Silver acetate is expensive and I used up to 1.5 mol equivalents! However, Zhang reported a catalytic version of this reaction, not with methyl vinyl ketone. Using his conditions I was able to reduce the silver acetate to 5 mol% simply by adding 5 mol% of triphenylphosphine which solublises the silver salt. The pyrrolidine came out in 50-60% yield. One minor problem, I got a wonderful silver mirrored flask as a side effect. I imagined this on a plant scale in a 1000L reactor it would not have made many friends.
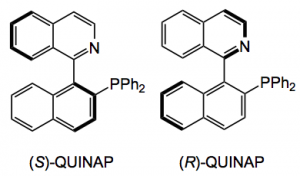
Of course the compound(s) I made were achiral and only one diastereoisomer was formed due to the cis addition. Chirality is always desirable so I looked at (R) and (S) – QUINAP as the phosphine source (5 mol%).
So this produced the pyrrolidine, after reaction with methyl vinyl ketone and the t-butyl aryl glycine imine in 40 -50% yield with an ee of 60-70% after reaction at -20°C, the result being the same with either the (S) or (R) the QUINAP. One re-crystallisation brought the ee up to 93% but further crystallisation did not improve this it just caused losses of material.
Now QUINAP is probably more expensive than silver acetate as its synthesis is very difficult and the resolution employs some horribly complex chiral palladium salt (whose structure I won’t attempt to draw here) and fractional crystallisation. So I examined many catalyst/ligand systems; the Trost ligand, Josiphos, quinine, Cu/ferrocene, Pflaz’s ligands, Carierra’s QUINAP variant, zinc triflates and so on. No luck, no reaction or reaction and no ee! So I was stuck with QUINAP. I forgot the chiral system and resorted to preparative chromatography using a Chiralpack column and supercritical CO2 as a mobile phase with some solvent system whose composition I forget. Anyway out came both enantiomers and eventually I had both of them in >10g quantities with ee’s of >98.8%.
So really this synthetic method is really great and works well. Since I was interested several newer publications (one from a South American group, this year in JOC) have reported really high %ee’s and I never got round to checking them out in my system, mainly because the project died. Typical for industry, you get a reasonable result and the project dies because of something going on elsewhere in the development chain.
Anyway a useful ASAP from the Waters’ group it will be nice to see how it develops in the coming publications.
9,804 total views, 3 views today