Tetravinylethylene (TVE) a molecule that has through and cross conjugation. Now that’s a compound that one may expect to have limited stability, on the contrary it is stable and for dust gathering periods of time according to Lindeboom, Willis, Paddon-Row and Sherburn form the Universities of New South Wales and the Australian National University Canberra. This group has just published a new synthesis of TVE and studied a few of its reactions.
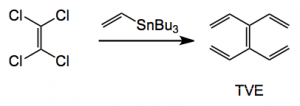
It was reported in 1966 by Skattebøl who made it in 0.1% yield in four steps from 1,5-cyclooctadiene. Since then there has apparently been no other reports of its synthesis or use until now. The current synthesis employs a Stille coupling of vinyl-tri-n-butylstannane and tetrachloroethylene employing Buchwald’s XPhos ligand.
Reagents: 5 mol equiv. of tri-n-butylvinylstannane, 0.040 mol equiv. Pd(OAc)2 , XPhos, 0.080 mol equiv., 60°C overnight, 64% yield.
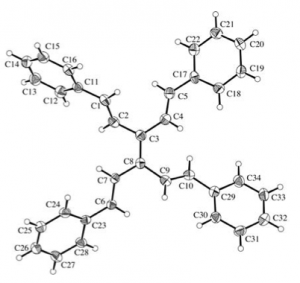
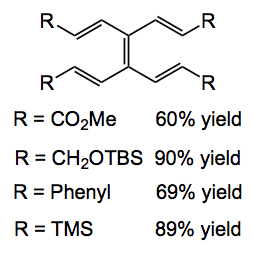
Kügelrohr distillation then delivers the pure TVE. In a nice “green touch” distillation of the tri-n-butyltin chloride into vinyl magnesium bromide regenerates the coupling starting material. TVE is stable in light and air for an extended period of time. The group also made a range of TVE analogues containing useful functionality and producing a nice x-ray of the tetra phenyl derivative:
So what does one do with this compound? Well the obvious answer is pericyclic reactions, in particular the Diels-Alder reaction. But it is not obvious how this would occur. TVE when heated or photolysed undergoes a 6π-electrocyclisation to compound 2, 2,3-divinyl-1,3-cyclohexadiene :
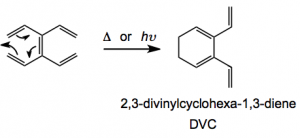 DVC is not quite so stable as its precursor and is best generated in situ. Both TVE and DVC undergo diene transmissive Diels-Alder reactions generating fused ring systems. The term diene transmissive Diels-Alder reaction was introduced by Tsuge etal in 1983 and refers to the “multiple Diels-Alder reactions of a cross-conjugated triene this reaction being able to transmit a diene functionality in order during the reaction. An example:
DVC is not quite so stable as its precursor and is best generated in situ. Both TVE and DVC undergo diene transmissive Diels-Alder reactions generating fused ring systems. The term diene transmissive Diels-Alder reaction was introduced by Tsuge etal in 1983 and refers to the “multiple Diels-Alder reactions of a cross-conjugated triene this reaction being able to transmit a diene functionality in order during the reaction. An example:
The product is obtained in 33% yield when the temperature is kept below 60°C. Even more impressive is the next reaction which forms 7 rings at once to give the product as a single diastereoisomer:
So with this nice little molecule and it’s derivatives it remains to be seen if it can, for example, find application in natural product synthesis, a specially the alkaloids. Perhaps we will see this in the future. Congratulations all on a nice piece of work.
![]()



It reminds me another insane molecule that has too many double bonds, 1,2,4,5-hexatetraene, Org. Synth. 1981, 60, 41. Also distillable and stable in fridge for months. Its Diels Alder product with acetylenedicarboxylic acid ester dimerizes to a paracyclophane. Good times.
Yep, these are great molecules.