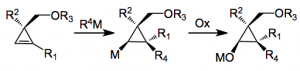
Cyclopropanes are interesting molecules, not just because of their synthesis but their reactivity. Several routes are available for their synthesis, even enantioselective ones. However a good method for the asymmetric preparation of substituted cyclopropanes, especially those with two adjacent quaternary centers is still lacking. This has changed with the report by Marek, from Israel, discussing an interesting route to these systems from substituted cyclopropenes, by a carbometallation/oxidation strategy:
The idea is that the stereocenter in the cyclopropene will induce diastereoselection in subsequent steps.
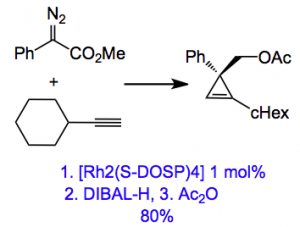
The chiral cyclopropene is easily synthesised by a carbene insertion into a triple bond catalysed by a chiral rhodium catalyst:
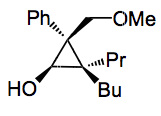
Now in the case where we have a methylether as R3 the addition of a cuprate and oxidation gives the following compound in reasonable yields and very high d.r for the isomer shown:
Conditions: 1. BuCuCNLi, -35°C, 30 min. 2. tBuOOLi, THF, -78°C, 3. acid. Yield 90%, d.r. 99:1:0:0.
Why the solvent change I’m not sure, perhaps one could just carry on with ether, or use THF at the start. I know ether forms peroxides, but so does THF. In any case: “…this stereoselectivity can be rationalised by the existence of a chelated transition state between the Lewis base of the ether and the organometallic species” we are told by the authors.
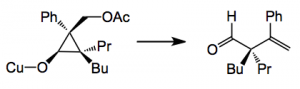
So what can we do with this. Cyclopropanes are well known for fragmentation/ring opening to give useful building blocks for synthesis. This is where the acetate comes in, it is a good leaving group and assists the fragmentation to a butenal, for example:
The yield for this compound is 66% and for the others they examined yields are in a similar range. Using a cyclohexyl substituent gave excellent e.r, 93:7. This value is not reported for the compound I drew, not even in the supplementary material!
So here is a useful method for small chiral building blocks. It would have been nice to have seen more varied examples apart from alkyl groups, but this may well be on it’s way. Nice methodology, it left me thinking about more useful compounds and applications in synthesis. I won’t divulge them here.
2,277 total views, 1 views today