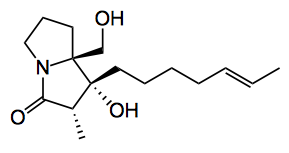
This week we have a natural product synthesis from Kim etal at the Seoul National University dealing with, amongst other things, dynamic kinetic resolution (DKR) and memory of chirality (MOC). The compound concerned is a pyrrolizidine alkaloid, (-)-penibruguieramine A:
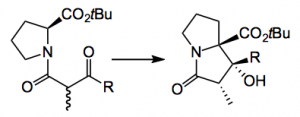
Their concept was to mimic the proposed biosynthetic route to this compound class starting with proline derivatives and the synthesis “features the asymmetric construction of stereocenters with essentially complete diastereo- and enantioselectivity in the absence of external chiral sources”. The key reaction is the intramolecular aldol reaction
“For the success of this MOC reaction, an axially chiral enolate (with an arbitrary enolate geometry) should be formed selectively over its enantiomer . Additionally, the chiral enolate must have a sufficiently large energy barrier against racemisation We anticipated the successful outcome of this reaction after considering the study reported by Stoodley et al. demonstrating the use of MOC in an aldol reaction of simpler proline derivatives. In that report, although the diastereoselectivity and yield were low, a certain degree of configuration retention was obtained at the chiral center of the proline moiety“. Further “The stereochemistry at the C2 position of aldol product can be introduced prior to the aldol reaction step. However, the C2 stereocenter of would not be retained over the course of the reaction due to facile enolization. We envisioned that if the DKR process occurred at the C2 position during the aldol reaction and the reaction proceeded via conformationally favored intermediate, then the C2 stereocenter would not require installation. Based on this assumption, the aldol substrate employed in this total synthesis was prepared as a diastereomeric mixture at the C2 position”. So they made an educated guess as to the outcome of this reaction!
The proline starting material was readily obtained (R = the actual side chain) in 5 steps and about 40% yield using “classical methods”. The best conditions for the aldol were now examined. Base, solvent, time and temperature are of course important factors in this reaction. The best base turned out to be sodium ethoxide (5 equiv. 9 hours at rt). This gave a 77% yield of the aldol product as a single diastereoisomer. The only other product was the dehydrated compound. Conversion to the natural product was achieved and it was obtained in >99% ee with the correct absolute configuration. This confirmed the effect of MOC during the reaction.
They separated the two methyl diastereoisomers and subjected them to the aldol conditions. Both gave the same product. NMR suggested that rapid epimerisation at that center was taking place “The diastereomeric ratio of the two isomers from the two reactions was 1.4:1 after 10 min, and the ratio remained constant. The ratio was the same prior to the separation, and may reflect the thermodynamic stability of the isomers. Based on these observations, we are confident that DKR occurred during the reaction“. Mechanistic experiments were conducted to confirm their original hypothesis.
A quick Google search of the MOC give lots of hits, the first up on the list are some useful lab presentations worth a read. This keyword is not often seen in publications, or if it is I may well have missed it. But in this ASAP the concept is used very effectively. In all a very interesting paper, well presented and a good read. Also something to remember.
2,123 total views, no views today