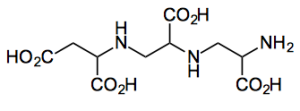
I spotted a paper by Capretta from the DeGroote Institute for Infectious Disease Research McMaster University,Hamilton, Canada. Presenting their approach to a metallo-β-lactamase inhibitor aspergillomarasmine A (AMA)
As we all know there are two types of prominent β-lactamases in pathogenic bacteria the serine and the metallo enzymes which have a zinc atom in the active site. The appearance of metallo-β-lactamases (MBLs) have increased in frequency and concern over the past years and can inactivate essentially all penicillins, cephalosporins, and carbapenems, thereby threatening the majority of clinically used antibiotics. Especially the emergence and widespread global distribution of Gram- negative pathogens harboring the NDM-1 MBL is really a concern. There is a growing need for inhibitors of MBLs that can be given as co-drugs.
Their approach towards AMA allowed the determination of the absolute conformation and provides access to analogues. It uses some nice aziridine building blocks derived from D or L-serine.
The reagents are what one would expect and the yield of the nosylated aziridine is around 65%. The protecting group swap was done to promote efficient ring opening, by the introduction of a more electron withdrawing group. It was known that the aspartic acid fragment was L. Opening of the nosylaziridine with a L-aspartic acid diester gave the expected product in 80% yield by simply stirring in THF at rt.
Removal of the nosyl group with thiophenol and further reaction with the aziridine and finally ester cleavage gives AMA. All 4 diastereoisomers were produced by this efficient route.
Ester hydrolysis was achieved selectively that is using Me3SnOH removes the methyl esters and TFA then cleaved the tBu. This also produced amounts of the cyclic anhydride-
So which isomer was the active one? From the authors “Assessment of the MBL-inactivation properties of the synthesised compounds with purified MBLs NDM-1 and VIM-2 revealed that all isomers of AMA had very similar properties to those of AMA derived from fermentation. This result was initially surprising but is consistent with a chelation model of enzyme inactivation, for which model building shows that different configurations do not significantly impact a predicted octahedral coordination of Zn2+. Further studies need to be carried out ………”
If this proposal is correct will be shown by further work. This approach is attractive and efficient. One can easily imagine medicinal chemistry programs to provide answers as well as more potent compounds.
3,002 total views, no views today