Benzyne is not a compound used in everyday synthesis but it appeared in this week’s ASAP from the group of Raminelli from the Universidade Federal de Saõ Paulo, Diadema, SP, Brazil. They were interested in synthesising some of the aporphine alkaloids which are biosynthetic derivatives of isoquinoline alkaloids and can be found in several families of plants and have interesting and important pharmacological properties. Exemplified by lysicamine, (±)-nuciferine, (±)-nornuciferine, (±)-zanthoxyphylline iodide, (±)-O-methylisothebaine, and (±)-trimethoxynoraporphine; all having the same core structure differing by the degrees of oxidation around the aromatic rings.
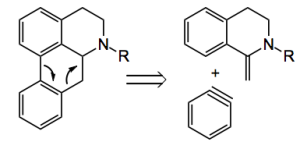
Reterosynthetic analysis reveals that the middle ring can be constructed by a [4+2] cycloaddition of benzyne and the 1-methyleneisoquinoline followed by a H migration:
So some conventional chemistry (not involving some transition metal) gives the isoquinoline in a few steps using a classical Bischler−Napieralski reaction producing the 1-methylisoquinoline in 90% yield.
The last step is done at -50°C with TFAA/pyridine and gives the methylene compound in 70% yield.
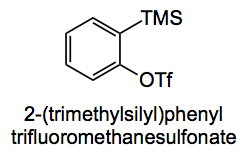
Conventional benzyne generation from anthranillic acid is probably too exciting a reaction to be carried out on a decent scale so Raminelli opted for benzyne generation under more milder conditions using
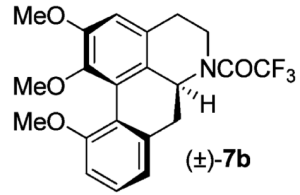
from which benzyne can be generated by treatment with caesium fluoride in acetonitrile at 80°C. The cycloaddition goes in 67% yield to give
Interestingly when 3-methoxy-2-(trimethylsilyl)phenyl trifluoro-methanesulfonate is used as the benzyne source the only compound formed is
So from the authors “Our approach involves the formation of aporphine cores by reactions between an isoquinoline derivative and silylaryl triflates (benzyne) promoted by CsF. The formation of aporphine cores proceeded in good yields, presumably through [4 + 2] cycloaddition reactions followed by hydrogen migrations. The chemistry disclosed represents an advance for the synthesis of aporphine alkaloids, providing aporphine cores under mild reaction conditions, and should find an application in the preparation of other aporphinoids”.
So some nice conventional chemistry here and good results using something as reactive as benzyne. Nice paper.
2,112 total views, 1 views today