Looking for that elusive starting material? You might find it described in this week’s ASAP from the Moeller group at Washington University in St. Louis. Sawdust, is the wonder source. Lot’s of sawdust out there, mainly existing between the ears of those who wish to …… Let’s not go there!
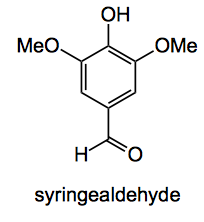
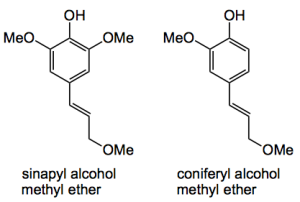
Sawdust is, of course, a source of lignin. That complicated structure of cross-linked polyphenol polymers which help to support your roof and keeps you sitting at your desk. So take your sawdust and put it in a pressure vessel and cook it with methanol. Remove the methanol and filter, concentrate the methanol chromatograph the residue and you isolate syringealdehyde in about 1% by mass from the raw sawdust or approximately 5% from the lignin in the sawdust (assuming that the sawdust was roughly 20% lignin). At the lower temperature and pressures both the methyl ether of sinapyl alcohol and the methyl ether of coniferyl alcohol are obtained in round 0.6%.
These compounds can be used as synthetic intermediates, which this paper also describes. However, “While the yield of the still unoptimised process is low, the ready availability of the sawdust starting material mitigates this issue, especially with the overall simplicity of the processes, the very high level of product selectivity obtained, and the fact that the remainder of the wood was recovered so that it could still be used for other purposes. This last point is important. For practical purposes, the solvolysis enables the selective extraction of desirable materials from the lignin in wood prior to processing the wood with more strenuous disassembly conditions that would more completely break apart the polymer and lead to mixtures of products” and “Not knowing the mechanism by which the products are generated complicates efforts to optimise the yield from the process. As mentioned above, most of the sawdust is recovered at the end of the solvolysis. The recovered sawdust does have a darker appearance than it did at the start of the reaction but otherwise appears unchanged. Resubmission of the sawdust to the methanolysis did lead to more of the desired product but afforded those products as a more difficult to separate mixture.”
Imagine your bosses face when you want scale this up and digest 100 kg of sawdust in his GMP cleaned plant! First a pre-treatment with benzene/ethanol. 100 kg would require 2 cubic meters benzene and 1 cubic ethanol. Then filter and dry and extract 100 kg dried material with 1 cubic methanol. Then filter concentrate and silica-gel chromatograph the residue with hexane/ethyl acetate. I don’t suppose this qualifies as a green chemistry process! In spite of the ready availability of sawdust.
Perhaps better leave the conversion of wood into useful materials to the woodworm which have the wonderful ability to destroy wood and convert it to protein in a very efficient manner.
2,782 total views, no views today