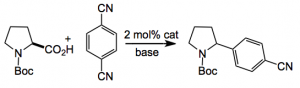
This week’s offering presents us with the preparation of a common pharmacophore from the amino acid biomass. and appeared in JACS written by MacMillan and Zuo of Princeton. The general strategy was seen as photoredox-mediated single-electron oxidation of the carboxylate functionality of an α-amino acid, followed by loss of CO2, to render an α-carbamoyl radical. Radical−radical coupling of this species with the radical anion derived from an arene coupling partner should forge the benzylic C−C bond to provide, following aromatization via expulsion of cyanide, the high-value benzylic amine motif. For example:
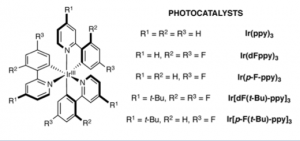
So you can see that the result is a molecule containing a useful moiety found in a lot of pharma. products. Yield is, of course, excellent and the reaction is illuminated with a normal 26 W light bulb. With yields being determined by NMR (again). Lots of substituted aryl amines were prepared so the scope is broad. The crucial part is of course the photocatalyst.
Now I could not find any reasonable reference to the preparation of these catalysts and it is probably well hidden in the text. But given that the authors are in the Merck Centre for Catalysis at Princeton they probably have bottles of the stuff lying around thus avoiding a 39 step synthesis to obtain it.
However an interesting approach to a useful pharmacophore. Worth remembering for all you medicinal chemists out there.
2,781 total views, 1 views today